| Citation: | ZHOU Fang-Liang, HE Lan, HE Dan, WANG Xian-Wen, SHI Hong-Jian, HE Ying-Chun, CAO De-Liang. Yi Qi Jie Du Decoction Inhibits Proliferation and Induces Apoptosis of Nasopharyngeal Carcinoma Stem Cells Through Mitochondrial Apoptosis Pathway[J]. Digital Chinese Medicine, 2019, 2(4): 219-226. DOI: 10.1016/j.dcmed.2020.01.003 |
Nasopharyngeal carcinoma (NPC) is the most common squamous cell carcinoma with a high incidence in Southeast Asia and North Africa, especially in South China [1, 2]. In China, the incidence of NPC is 20/100 000, with 60 000 new NPC patients diagnosed every year; these rates are increasing exponentially [3]. The pathogenesis of NPC involves multiple factors, such as Epstein–Barr virus infection, genetic factors, and environmental impacts [4]. Radiotherapy and chemotherapy are the most effective treatments for NPC [5]. Although these therapies have achieved good therapeutic effects, they do not completely suppress NPC development and progression, relapse, and early metastasis [6]. Therefore, it is required to seek novel molecular therapeutic targets to develop a curative treatment.
Cancer stem cells (CSCs) may be related to recurrence and metastasis of cancer [7]. CSCs are defined as a subgroup of tumorigenic cells that possess stem cell-like characteristics, such as pluripotency, self-renewal, and an overall innate resistance to conventional chemotherapeutic agents [8, 9]. In the past decade, CSCs have been demonstrated to exist in various malignant tumors, such as breast cancer [10], brain tumors [11], colon cancer [12], ovarian carcinoma [13], gastric cancer [14] and many others. In addition, several studies identified CSCs from human NPC cells and tissues [15-17]. Therefore, targeting therapy towards CSCs in NPC is expected to be an effective method to eradicate NPC and prevent recurrence. Activating pro-apoptotic pathways is one of the main therapeutic methods to eradicate CSCs [7].
Yi Qi Jie Du Decoction(YQJDD) is an empirical formula researched and applied by our group over the course of 20 years. YQJDD has been shown to have a therapeutic effect in clinical treatment of NPC [18-22]. It consists of Astragali Radix (Huang Qi, 黄芪), Codonopsis Radix (Dang Shen, 党参), Trichosanthis Radix (Tian Hua Fen, 天花粉), Herba Hedyotis (Bai Hua She She Cao, 白花蛇舌草), Poria (Fu Ling, 茯苓), GLycyrrhizae Radix Ex Rhizoma (Gan Cao, 甘草), and Coptidis Rhizoma (Huang Lian, 黄连). Previous studies demonstrated that YQJDD can inhibit Epstein–Barr virus infection, modulate interleukin-2, as well as reduce telomerase activity [23-26]. In addition, it can inhibit the proliferation and induce death of NPC cells by modulating nuclear transcription factors, regulating the G0/G1 phase of cell cycle, and inducing the expression of apoptosis genes [27-33]. However, the precise effect of YQJDD on NPC stem cells (NPC-SCs) remains to be elucidated.
The objective of this study was to investigate the mechanism of YQJDD effects on NPC-SCs. YQJDD clearly inhibited proliferation and induced apoptosis of NPC-SCs through the mitochondrial apoptosis pathway. The findings further clarified the mechanism of the influence of YQJDD on NPC-SCs and provided novel perspectives for the prevention and treatment of cancer with traditional Chinese medicine (TCM).
YQJDD consisted of the following components: Astragali Radix (Huang Qi, 黄芪), Codonopsis Radix (Dang Shen, 党参), Trichosanthis Radix (Tian Hua Fen, 天花粉), Herba Hedyotis (Bai Hua She She Cao, 白花蛇舌草), Poria (Fu Ling, 茯苓), GLycyrrhizae Radix Ex Rhizoma (Gan Cao, 甘草), and Coptidis Rhizoma (Huang Lian, 黄连). All materials were purchased from the First Hospital of Hunan University of Chinese Medicine (Changsha, China). The materials were mixed in a respective ratio of 20∶10∶10∶20∶10∶6∶10 (dry weight). The mixture was soaked overnight in ten times the volume of distilled water and boiled at 100 °C for 30 min. The drug solution was then separated for further use and the sediment mixed with three times its volume of distilled water and boiled at 100 °C for 20 min. All of the drug solutions were refluxed for 4 h in a Soxhlet extractor, cooled under vacuum, dried to powder, and finally dissolved in a storage solution with a concentration of 10 mg/mL in NPC-SCs medium (equivalent of 100 mg/mL dry weight of raw materials) [34].
The CNE2 human NPC cell line was purchased from Sun Yat-sen University (Guangzhou, China). Cells were cultured in Roswell Park Memorial Institute (RPMI) 1 640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA), 2 mM L-glutamine, and 100 U/mL penicillin-streptomycin. CNE2 CSCs (CNE2-SCs) were enriched in a serum-free culture system [35]. Briefly, CNE2 cells were mixed at a density of 1 × 105 cells/mL in serum-free RPMI (SFR) containing B27 (1∶50, Gibco, Carlsbad, CA, USA), 20 ng/mL of basic fibroblast growth factor (bFGF, Gibco), 20 ng/mL epidermal growth factor (Gibco), and 5 μg/mL routine insulin (Invitrogen, Carlsbad, CA, USA) in ultra-low adhesion six- well plates (Corning Life Sciences, Acton, MA, USA). Spheroids were passaged approximately every 8 days. The spheroids were dissociated enzymatically with Accutase (Gibco) into single cells and their capacity to generate secondary and tertiary gastrospheroids was examined by counting the spheroids larger than 50 μm at the time of passage in 20 distinct microscopic fields of view (Olympus Corp, Tokyo, Japan). The sphere-forming efficiency was calculated by determining sphere-forming efficiency (‰) = (number of spheroids / number of plated cells) × 100. The cells were cultured at 37 °C in an atmosphere of 5% CO2.
Cell proliferation was determined by counting viable cells with a Cell Counting Kit-8 (CCK-8; Amresco, Solon, OH, USA). Spheroid cells were trypsinized and plated at a density of 3 000 cells/well in a 96-well plate and cultured overnight. The cells were then treated with various concentrations of YQJDD (0, 0.5, 1, 2 and 4 mg/mL). After 24, 48 and 72 h, the culture solution was discarded, and 100 μL CCK8 was added to each well and incubated for 1.5 h at 37 °C. The absorbance/optical density (OD value) at 450 nm was determined using a microplate reader. The cell viability was calculated as (ODtreatment – ODblank)/(ODcontrol – ODblank) × 100. All cell viability assays were performed at least 3 times.
Mitochondrial stability was assessed using an ΔΨm assay kit with JC-1 staining (Cayman Chemical, Ann Arbor, MI, USA). CNE2-SCs were plated at a density of 5 × 105 cells/well in six-well plates with 2 mL SFR per well and cultured overnight. Next, the cells were treated with 2 mg/mL YQJDD. After treatment for 0, 24, 36 and 48 h, 200 µL of JC-1 Staining Solution was added to each well and incubated for 20 min in the dark at 37 °C. Subsequently, the cells were washed twice by centrifugation (400 × g, 5 min) with the assay buffer. The ΔΨm was detected using a fluorescence microscope (Olympus Corp.) using excitation/emission wavelengths of 540/570 nm and 485/535 nm. Cell apoptosis rates were also calculated.
After treatment, cells were lysed in RIPA buffer with 10% phenylmethylsulfonyl fluoride and centrifuged at 1 000 × g for 5 min at 4 °C. Protein extracts were quantified using a BCA protein assay kit (Lianke, Hangzhou, China). Equal amounts of proteins (30 μg or 60 μg per lane) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% fat-free milk in Tris buffered saline-Tween (TBST) at room temperature for 1 h, and then incubated with primary antibodies. The antibodies and dilutions were: anti-ATP-binding cassette super-family G member 2 (ABCG2, 1∶1 000), anti-CD44 (1∶1 000), anti-aldehyde dehydrogenase 1/2 (ALDH1/2; 1∶1 000), anti-cleaved caspase-3 (1∶500), anti-cleaved caspase-7 (1∶1 000), anti-cleaved caspase-8 (1∶1 000), anti-cleaved caspase-9 (1∶1 000), anti-cleaved poly-ADP ribose polymerase (PARP, 1∶500), anti-P21 (1∶1 000), anti-P53 (1∶1 000), and anti-Survivin (1∶1 000) at 4 °C overnight (all from Cell Signaling Technology, Beverly, MA, USA). After primary antibody incubation, membranes were washed three times in TBST and then incubated with secondary antibody (1∶5 000, LI-COR, Lincoln, NE, USA) for 1 h at room temperature. After washing, the signals were visualized by the Odyssey Infrared Imaging System (LI-COR). Endogenous beta-actin was used for normalization.
SPSS 17.0 software (SPSS, Chicago, IL, USA) was used for statistical analyses. All experiments were performed at least 3 times. Results are presented as the mean ± SD. Statistical analyses were conducted using ANOVA or Student’s t-test. The LSD method was used for multiple comparisons. Statistically significant differences were indicated by P < 0.05.
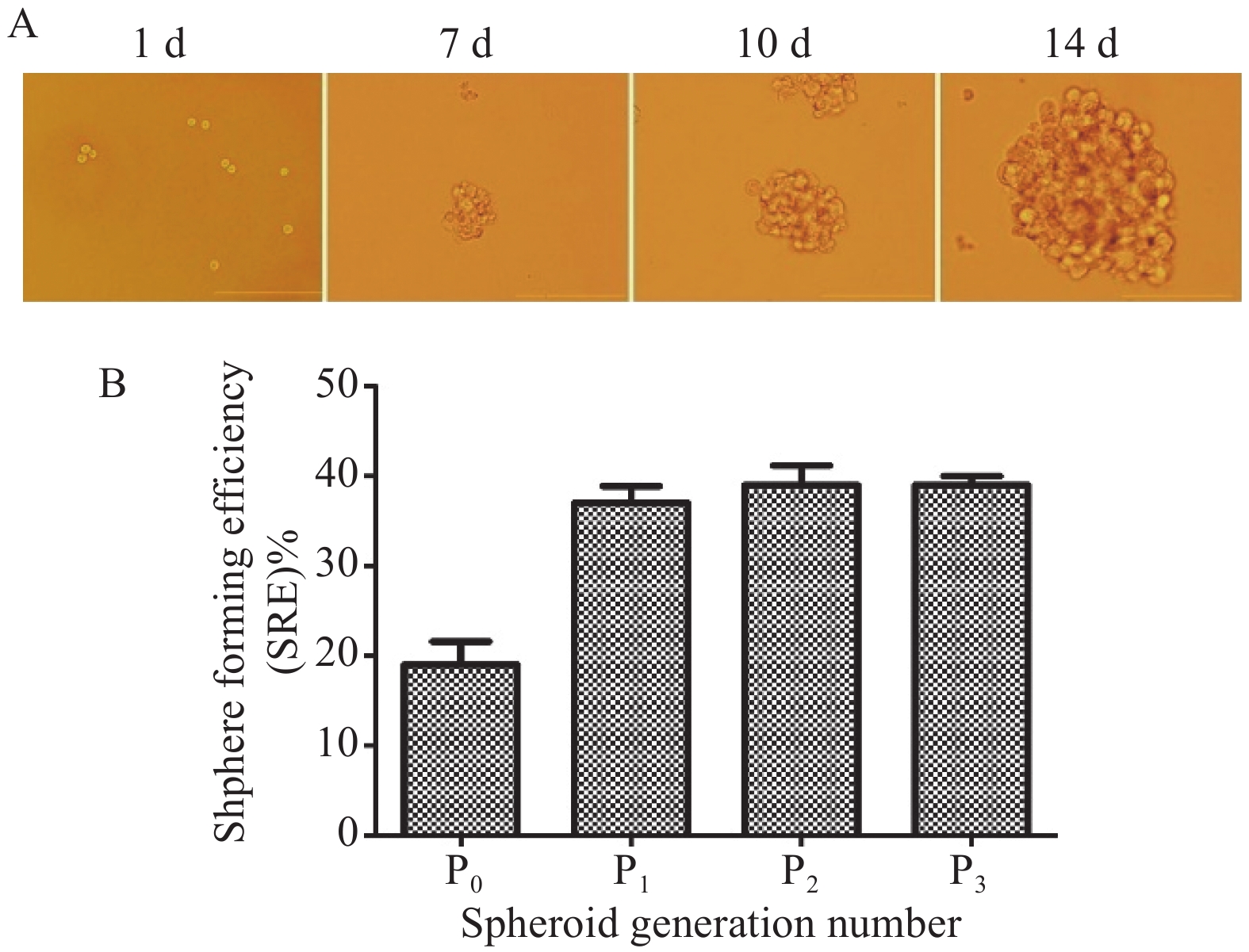
Initially, we generated NPC-SCs from the CNE2 human NPC cell line by primary culture of floating spheroids. Since CSCs are capable of self-renewal and producing next generation spheres [8, 35], we checked whether these NPC-SCs possessed these characteristics. The NPC-SCs grew as non-adherent, three-dimensional clusters that increased in size over time (Figure 1A). The sphere-forming efficiency of NPC-SCs (P1, P2 and P3) was significantly higher than the CNE2 cells (P0) (Figure 1B). These results provided definitive evidence that the spheroids isolated from the serum-free culture system had characteristics of CSCs. These spheroids were designated CNE2-SCs and were used for further identification.
Differential expression of cell surface and functional markers has been described between monolayer cells and CSCs [36, 37]. ABCG2 transports various molecules through extracellular and intracellular membranes, and is associated with the phenotype of CSCs [38]. CD44 is a cell surface glycoprotein that has been reported as a cell surface marker for some CSCs and is involved in cell adhesion and migration [37, 39]. CD44 also increases the expression of ALDH in NPCs and is associated with stem cell-like properties [36, 40]. Therefore, we checked whether CNE2-SCs highly expressed these CSCs marker proteins. Western blot analysis showed that the expressions of CD44, ABCG2 and ALDH1/2 were up-regulated in CNE2-SCs compared with monolayer CNE2 cells (Figure 2). These results suggested that CNE2-SCs enriched in serum-free culture condition express the stem cell-related markers.
The CNE2-SCs were treated with various concentrations (0, 0.5, 1, 2 and 4 mg/mL) of YQJDD for 0, 24, 48 and 72 h. The CCK-8 assay revealed that the proliferation of CNE2-SCs was significantly inhibited by YQJDD in dose- and time-dependent manners compared to the control group (Figure 3). The IC50 was approximately 2 mg/mL at 72 h. The YQJDD concentration of 2 mg/mL was used in the subsequent experiments.
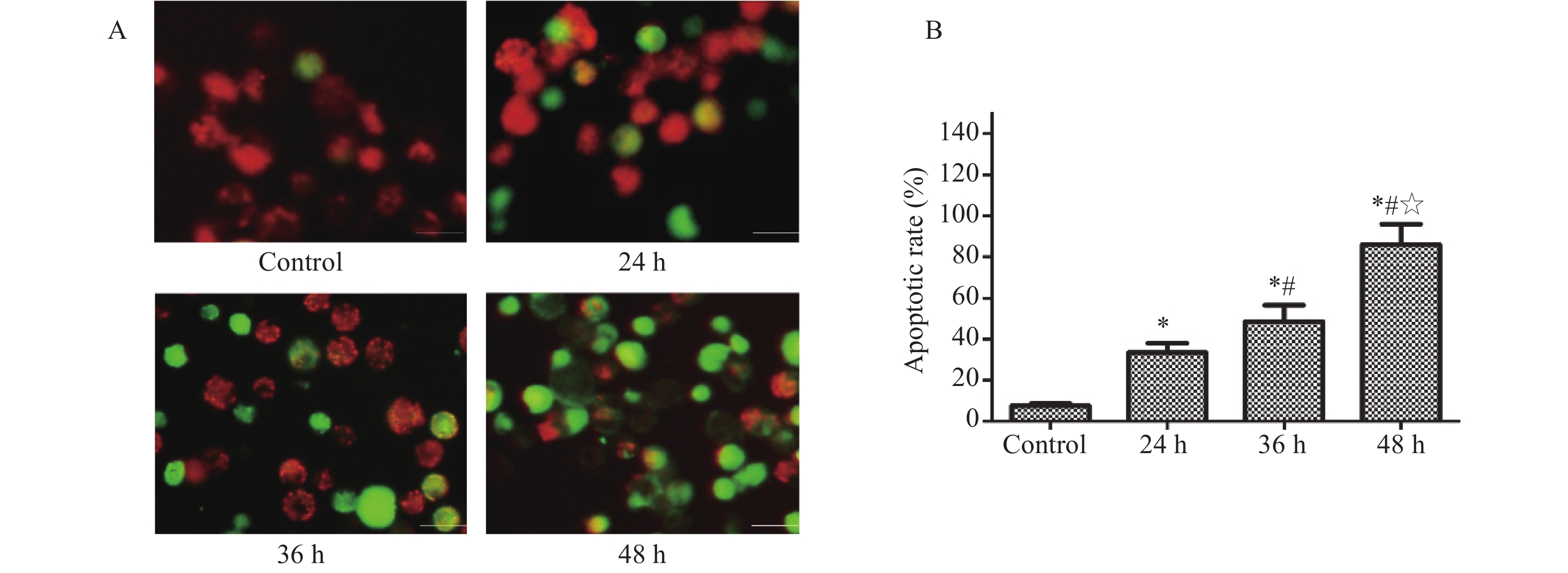
JC-1 staining revealed increasing fluorescence conversion from red to green with prolonged treatment with YQJDD (Figure 4A). These results indicated that YQJDD lowered the ΔΨm of CNE2-SCs in a time-dependent manner. Since a decrease in ΔΨm can be a sign of early apoptosis, we calculated the apoptotic rate in the cells following treatment. YQJDD significantly induced apoptosis of CNE2-SCs in a time-dependent manner (Figure 4B).
In order to elucidate the effects of YQJDD on CNE2-SCs, the cells were treated with 2 mg/mL of YQJDD for 0, 24, 36 and 48 h, and western blotting analysis was used to investigate the expressions of apoptosis-associated proteins. Expressions of P53, P21, cleaved caspases-3, -7 and -9, and PARP were up-regulated, the expression of Survivin was down-regulated, while the expression of cleaved caspase-8 was unchanged in the YQJDD group compared to the control group (Figure 5). These findings indicated that YQJDD may induce apoptosis in CNE-SCs via a mitochondrial apoptotic pathway.
CSCs are hypothesized to be involved in the metastasis and recurrence of tumors [8]. In recent years, CSCs from human NPC cells and tissues have been isolated and identified [35, 36, 39, 41, 42]. Targeted NPC-SCs may be a new therapeutic strategy for NPC. Since CSCs are extremely rare, the main difficulty in studying CSCs is how to identify and isolate them from a large number of tumor cells. At present, the common methods to isolate CSCs include flow cytometry based on cell surface markers, bioenzymes and specific dyes, serum-free culture system, and immunomagnetic separation [35, 43]. In this study, we chose a serum-free culture system since it can collect CSCs quickly and efficiently. Spherical cells possessed a self-renewal ability, were able to generate new spheres, and overexpressed stem-relevant genes (ABCG2, CD44 and ALDH1/2) compared with the parental cells. These results suggested that the collected spherical cells have the characteristics of CSCs and can be used in the later experiments.
YQJDD, a Chinese herbal formula, has been shown to have anti-NPC activity in several studies [18, 24, 25, 28]. However, the precise interaction mechanism of YQJDD with NPC-SCs has been unclear. In this study, experiments were undertaken to explore the influence of YQJDD on NPC-SCs. The results indicated that YQJDD inhibits NPC-SCs growth in both dose-and time-dependent manners. In addition, since the fluorescence change from red to green after JC-1 staining can determine if the ΔΨm (a sign of early apoptosis) is changed [44], we used JC-1 staining to observe whether YQJDD can induce the apoptosis of CNE2-SCs. The results showed that ΔΨm decreased (as green fluorescence intensity was enhanced) and the early apoptosis was induced after CNE2-SCs were treated with YQJDD.
There are 2 main pathways to apoptosis: an intrinsic pathway (mitochondrial pathway) and an extrinsic pathway (cell membrane surface death receptor mediated pathway) [45]. To further confirm the mechanism of YQJDD on CNE2-SCs, we evaluated the effect of YQJDD on apoptosis-associated proteins. In the extrinsic pathway, the extracellular pro-apoptotic ligands bind to death receptors [46]. The complex composed of procaspase-8, -10 and Fas-associated death domain can initiate the apoptosis process by activating procaspase-8 and -10, and cleaving procaspase-3 [47, 48]. In the present study, we found that the expression of cleaved caspase-8 was unchanged after treatment with YQJDD, indicating that YQJDD likely did not induce apoptosis via the extrinsic pathway. The intrinsic pathway (the mitochondrial pathway) is triggered by a variety of stress signals that induce cellular damage [49]. This pathway can improve mitochondrial membrane permeability and allow the efflux of apoptotic proteins, which lead to apoptosis by activating caspases-3, -6, -7 and -9 [50]. In this study, we found that the expression of cleaved caspase-3, -7 and -9 in CNE2-SCs was significantly elevated when treated with 2 mg/mL YQJDD for 36 h. Therefore, we can infer that YQJDD induced apoptosis of NPC-SCs through the mitochondrial apoptosis pathway. Moreover, the expression of P53 and P21 (transcription targets of P53) were also up-regulated after YQJDD treatment. P53 is reported to play key roles in the mitochondrial apoptosis pathway [51, 52]. Therefore, we speculate that YQJDD may induce the apoptosis of NPC-SCs through P53-dependent mitochondrial apoptosis pathways. However, further elucidation of molecular mechanisms is needed and studies in vivo are indispensable to further prove the effect of YQJDD on NPC-SCs.
In summary, the results presented here show that YQJDD inhibits the growth of NPC-SCs and induces apoptosis of NPC-SCs via an intrinsic apoptosis pathway. In addition, P53 may play a vital role in this process. The effects of YQJDD on NPC-SCs have not been described previously. These results provide new insights for the prevention and treatment of tumors using traditional Chinese medicine.
We thank for the funding support from the National Natural Science Foundation of China (No. 81573721, No. 81874408 and No. 81973914), the Natural Science Foundation of Hunan (No. 2017JJ3246 and No. 2019JJ40216) and Excellent Youth Fund of Hunan Provincial Education Department (No. 19B439).
| [1] |
LIN CY, CAO SM, CHANG ET, et al. Chinese nonmedicinal herbal diet and risk of nasopharyngeal carcinoma: a population-based case-control study. Cancer, 2019, 125(24): 4462–4470.
|
| [2] |
CHAN JYW, HUANG WB, LIU DL. Reply to human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in southern China. Cancer, 2019, 125(1): 161–162.
|
| [3] |
CHEN W, ZHENG R, BAADE PD, et al. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians, 2016, 66(2): 115–132. doi: 10.3322/caac.21338
|
| [4] |
NOR HASHIM NA, RAMZI NH, VELAPASAMY S, et al. Identification of genetic and non-genetic risk factors for nasopharyngeal carcinoma in a Southeast Asian population. Asian Pacific Journal of Cancer Prevention: APJCP, 2012, 13(12): 6005–6010. doi: 10.7314/APJCP.2012.13.12.6005
|
| [5] |
LIU F, LUO T, JIN T, et al. Advantages of using reduced-volume intensity modulated radiation therapy for the treatment of nasopharyngeal carcinoma: a retrospective paired study. BMC Cancer, 2019, 19(1): 554. doi: 10.1186/s12885-019-5774-2
|
| [6] |
XU T, TANG J, GU M, et al. Recurrent nasopharyngeal carcinoma: a clinical dilemma and challenge. Current Oncology, 2013, 20(5): e406–419. doi: 10.3747/co.20.1456
|
| [7] |
HE YC, ZHOU FL, SHEN Y, et al. Apoptotic death of cancer stem cells for cancer therapy. International Journal of Molecular Sciences, 2014, 15(5): 8335–8351. doi: 10.3390/ijms15058335
|
| [8] |
FRAME FM, MAITLAND NJ. Cancer stem cells, models of study and implications of therapy resistance mechanisms. Advances in Experimental Medicine and Biology, 2011, 720: 105–118.
|
| [9] |
DALERBA P, CHO RW, CLARKE MF. Cancer stem cells: models and concepts. Annual Review of Medicine, 2007, 58: 267–284. doi: 10.1146/annurev.med.58.062105.204854
|
| [10] |
LIU S, DONTU G, WICHA MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Research: BCR, 2005, 7(3): 86–95. doi: 10.1186/bcr1021
|
| [11] |
SINGH SK, HAWKINS C, CLARKE ID, et al. Identification of human brain tumour initiating cells. Nature, 2004, 432(7015): 396–401. doi: 10.1038/nature03128
|
| [12] |
CHEN X, WEI B, HAN X, et al. LGR5 is required for the maintenance of spheroid-derived colon cancer stem cells. International Journal of Molecular Medicine, 2014, 34(1): 35–42. doi: 10.3892/ijmm.2014.1752
|
| [13] |
STEWART JM, SHAW PA, GEDYE C, et al. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(16): 6468–6473. doi: 10.1073/pnas.1005529108
|
| [14] |
HAJIMORADI M, MOHAMMAD HASSAN Z, EBRAHIMI M, et al. STAT3 is overactivated in gastric cancer stem-like cells. Cell Journal, 2016, 17(4): 617–628.
|
| [15] |
BIAN S, WANG Z, CHEN Y, et al. SPLUNC1 and MLL3 regulate cancer stem cells in nasopharyngeal carcinoma. Journal of BUON: Official Journal of the Balkan Union of Oncology, 2019, 24(4): 1700–1705.
|
| [16] |
WARDHANI LK, KENTJONO WA, ROMDHONI AC. Association between dose and duration of cisplatin exposure with cytotoxicity effect on nasopharyngeal carcinoma stem cell. Indian Journal of Otolaryngology and Head and Neck Surgery: Official Publication of the Association of Otolaryngologists of India, 2019, 71(Suppl 1): 373–377.
|
| [17] |
EDITORS PO. Expression of concern: identification of CD24 as a cancer stem cell marker in human nasopharyngeal carcinoma. PloS One, 2019, 14(1): e0210304. doi: 10.1371/journal.pone.0210304
|
| [18] |
JIANG ZC, TIAN DF, FAN JY. Effects of Qi-Boosting Toxin-Resolving Formula on CD4+CD25+ Regulatory T cells and Th17 cells of patients with middle to late staged nasopharyngeal carcinoma. Chinese Journal of Information on Traditional Chinese Medicine, 2014, 2(2): 23–26+31.
|
| [19] |
LUO JJ. Study the inhibition of proliferation, invasion, migration and induction of apoptosis in nasopharyngeal carcinoma cells by aqueous extract of Yiqi Jiedu formula combined with Salinomycin [dissertation]: Hunan University of Chinese Medicine, 2016.
|
| [20] |
TANG FQ, LIANG RC, TIAN DF, et al. The inhibitory effect of YIQIJIEDU granule on the transformation of embryo nasopharyngeal epithelia induced by DNP. Journal of Traditional Chinese Medicine University of Hunan, 2001, 21(3): 18–20.
|
| [21] |
TIAN DF, TANG FQ, CHEN XY, et al. A Clinical observation on the inhibitory effect of Yiqijiedu granules to the infection activity of EBV in population highly susceptible to NPC. Journal of Traditional Chinese Medicine University of Hunan, 2000, 20(3): 47–49.
|
| [22] |
WANG DH, TIAN DF. Clinical studied on the Therapeutic effects of combined therapy with radiation and Qi-boosting toxin-resolving granule on nasopharynnfeal carcinoma. Journal of Traditional Chinese Medicine University of Hunan, 2006, 26(1): 36–37.
|
| [23] |
TANG AG, TIAN DF, YI YL, et al. Inhibitory growth of nasopharyngeal carcinoma cells by YIQIJIEDU granule via down regulating telomerase. China Journal of Modern Medicine, 2005, 15(1): 53–55.
|
| [24] |
TANG FQ, TIAN DF, DENG FL, et al. Effect of Yiqijiedu granule on the implanted-tumor protein expression in nasopharyngeal carcinoma. Journal of Central South University (Medical Science), 2004, 29(5): 577–582.
|
| [25] |
TANG FQ, TIAN DF, YI H, et al. An Experimental study on the inhibitory effect of Yiqijiedu Tablets to telomerase and telomerase RNA in nasopharyngeal carcinoma cells. Journal of Traditional Chinese Medicine University of Hunan, 2000, 20(1): 15–17+72.
|
| [26] |
ZHOU XJ, DU GY, SUN YF, et al. Impact of Biyan Jiedu Keli on TNF-α and IL-2 levels in EB viral infection. World Journal of Integrated Traditional and Western Medicine, 2009, 4(10): 721–723.
|
| [27] |
CHENG XH, TIAN DF, YU R, et al. Effect of Yiqi Jiedu decoction on apoptosis and proliferating in developing process of carcinogenesis in rats. Journal of Traditional Chinese Medicine University of Hunan, 2009, 29(2): 10–13.
|
| [28] |
HE YC, LIU DD, SHANG YF. Effects of Qi-Boosting Toxin-Resolving Formula on cell migration and c-myc and p-Ezrin transcriptional activities of CNE2 cells in vitro. Chinese Journal of Otorhinolaryngology In Integrative Medicine, 2010, 18(1): 14–17.
|
| [29] |
HU BY, TIAN DF, HE YC. Studies on the effects of Qi-Boosting Toxin-Resolving decoction on proliferation of nasopharyngeal carcinoma cell. Journal of Clinical Otorhinolaryngology Head and Neck Surgery, 2009, 23(12): 558–560.
|
| [30] |
HU M. A Study on the intervening effects of effective fractions from Qi-Boosting Toxin-Resolving Formula with dendritic cells in the tumor microenvironment of NPC [dissertation]: Hunan University of Chinese Medicine, 2012.
|
| [31] |
LIU DD, HE YC, SHANG YF. Intervening mechanisms with cell migration underlying the inhibiting effect of Qi-Boosting Toxin-Resolving Formula on the metastatic potentiality of NPC cells. Chinese Journal of Otorhinolaryngology In Integrative Medicine, 2010, 18(1): 18–21.
|
| [32] |
JING H, JIE L, BINGYAN X, et al. Effects of Yiqi Jiedu Formula on proliferation of nasopharyngeal carcinoma CNE2 cells. Journal of Hunan University of Chinese Medicine, 2019, 39(8): 943–947.
|
| [33] |
MEI H, FANGLIANG Z, LAN S, et al. Effect of Qi-Boosting Toxin-Resolving Granules and cisplatin on transplanted tumor of nasopharyngeal carcinoma and balance of Foxp3/ROR-γt. Chinese Traditional and Herbal Drugs, 2019, 50(9): 2121–2126.
|
| [34] |
SHEN J, ZHU Y, HUANG K, et al. Buyang Huanwu Decoction attenuates H2O2-induced apoptosis by inhibiting reactive oxygen species-mediated mitochondrial dysfunction pathway in human umbilical vein endothelial cells. BMC Complementary and Alternative Medicine, 2016, 16: 154. doi: 10.1186/s12906-016-1152-7
|
| [35] |
WU SL, LI YJ, LIAO K, et al. 2-Methoxyestradiol inhibits the proliferation and migration and reduces the radioresistance of nasopharyngeal carcinoma CNE-2 stem cells via NF-kappaB/HIF-1 signaling pathway inactivation and EMT reversal. Oncology Reports, 2017, 37(2): 793–802. doi: 10.3892/or.2016.5319
|
| [36] |
WEI P, NIU M, PAN S, et al. Cancer stem-like cell: a novel target for nasopharyngeal carcinoma therapy. Stem Cell Research & Therapy, 2014, 5(2): 44.
|
| [37] |
TAKAISHI S, OKUMURA T, TU S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells, 2009, 27(5): 1006–1020. doi: 10.1002/stem.30
|
| [38] |
ZHANG H, LIU W, FENG X, et al. Identification of ABCG2 (+) cells in nasopharyngeal carcinoma cells. Oncology Reports, 2012, 27(4): 1177–1187. doi: 10.3892/or.2011.1618
|
| [39] |
JANISIEWICZ AM, SHIN JH, MURILLO-SAUCA O, et al. CD44 (+) cells have cancer stem cell-like properties in nasopharyngeal carcinoma. International Forum of Allergy & Rhinology, 2012, 2(6): 465–470.
|
| [40] |
HUANG EH, HYNES MJ, ZHANG T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Research, 2009, 69(8): 3382–3389. doi: 10.1158/0008-5472.CAN-08-4418
|
| [41] |
LI YJ, WU SL, LU SM, et al. (-)-Epigallocatechin-3-gallate inhibits nasopharyngeal cancer stem cell self-renewal and migration and reverses the epithelial-mesenchymal transition via NF-kappaB p65 inactivation. Tumour Biology: the Journal of the International Society for Oncodevelopmental Biology and Medicine, 2015, 36(4): 2747–2761. doi: 10.1007/s13277-014-2899-4
|
| [42] |
WANG J, GUO LP, CHEN LZ, et al. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Research, 2007, 67(8): 3716–3724. doi: 10.1158/0008-5472.CAN-06-4343
|
| [43] |
BOO L, HO WY, ALI NM, et al. MiRNA transcriptome profiling of spheroid-enriched cells with cancer stem cell properties in human breast MCF-7 cell line. International Journal of Biological Sciences, 2016, 12(4): 427–445. doi: 10.7150/ijbs.12777
|
| [44] |
CASTEDO M, FERRI K, ROUMIER T, et al. Quantitation of mitochondrial alterations associated with apoptosis. Journal of Immunological Methods, 2002, 265(1-2): 39–47. doi: 10.1016/S0022-1759(02)00069-8
|
| [45] |
TAYLOR RC, CULLEN SP, MARTIN SJ. Apoptosis: controlled demolition at the cellular level. Nature Reviews Molecular Cell Biology, 2008, 9(3): 231–241. doi: 10.1038/nrm2312
|
| [46] |
LAVRIK I, GOLKS A, KRAMMER PH. Death receptor signaling. Journal of Cell Science, 2005, 118(Pt 2): 265–267.
|
| [47] |
SAYERS TJ. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunology, Immunotherapy: CII, 2011, 60(8): 1173–1180. doi: 10.1007/s00262-011-1008-4
|
| [48] |
KISCHKEL FC, LAWRENCE DA, TINEL A, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. The Journal of Biological Chemistry, 2001, 276(49): 46639–46646. doi: 10.1074/jbc.M105102200
|
| [49] |
PATERGNANI S, MISSIROLI S, MARCHI S, et al. Mitochondria-associated endoplasmic reticulum membranes microenvironment: targeting autophagic and apoptotic pathways in cancer therapy. Frontiers in Oncology, 2015, 5: 173.
|
| [50] |
HOCKENBERY DM. Targeting mitochondria for cancer therapy. Environmental and Molecular Mutagenesis, 2010, 51(5): 476–489. doi: 10.1002/em.20552
|
| [51] |
GALLUZZI L, MORSELLI E, KEPP O, et al. Targeting p53 to mitochondria for cancer therapy. Cell Cycle, 2008, 7(13): 1949–1955. doi: 10.4161/cc.7.13.6222
|
| [52] |
YAN M, QIAN YM, YUE CF, et al. Inhibition of histone deacetylases induces formation of multipolar spindles and subsequent p53-dependent apoptosis in nasopharyngeal carcinoma cells. Oncotarget, 2016, 7(28): 44171–44184.
|
| 1. | Zhou F., Wang W., Xu R. et al. Unraveling the mechanism of Yiqi Jiedu formula against nasopharyngeal carcinoma: An investigation integrating network pharmacology, serum pharmacochemistry, and metabolomics. Journal of Ethnopharmacology, 2024, 319 DOI:10.1016/j.jep.2023.117343 |
| 2. | Lan H., Fang-Liang Z., Pan Z. et al. Yi Qi Jie Du Formula and Salinomycin Combination Treatment Mediates Nasopharyngeal Carcinoma Stem Cell Proliferation, Migration and Apoptosis via CD44/Ras Signaling Pathway. Digital Chinese Medicine, 2020, 3: 297-308. DOI:10.1016/j.dcmed.2020.12.008 |