| Citation: | Citation: RONG XQ, WU MS, XIN XZ, et al. Efficacy and safety of Jiuhua hemorrhoid suppository plus diosmin for the treatment of hemorrhoid hemorrhage: a multicenter, randomized, and controlled trial. Digital Chinese Medicine, 2023, 6(4): 467-476. DOI: 10.1016/j.dcmed.2024.01.009 |
To compare the efficacy and safety of combining diosmin with Jiuhua hemorrhoid suppository versus diosmin alone for the treatment of hemorrhoid hemorrhage.
The Jiuhua hemorrhoid suppository study was conducted in 10 medical centers across China from April 1, 2019 to June 30, 2020. Patients with hemorrhoid bleeding were randomized in a ratio of 1 : 1 to either receive Jiuhua hemorrhoid suppository and diosmin tablets (the study group) or diosmin tablets alone (the control group). The suppository was used once a day after defecation or at bedtime after rinsing the anus with warm water. Diosmin tablets were administered only once a day (0.9 g). The primary endpoint of the study was the assessment of hemorrhoid bleeding relief 7 ± 2 days after treatment, classified as “very effective” “effective” and “ineffective”. The secondary endpoint included the evaluation of pain alleviation using the visual analogue scale (VAS, with scores ranging from 0 to 10) and edema (with scores ranging from 0 to 3). The safety of the two treatment regimens was evaluated 14 ± 2 days after drug administration.
The full analysis set (FAS) comprised 107 participants in the study group and 111 in the control group, while the per-protocol set (PPS) included 106 participants in the study group and 111 in the control group. In terms of hemorrhoid bleeding, the proportion of very effective and effective cases in the study group were significantly higher than that in the control group [106 (99.06%) vs. 91 (81.98%), P < 0.0001] in the FAS, and the PPS results [105 (99.06%) vs. 91 (81.98%), P < 0.0001] were comparable to the FAS results. The pain VAS scores at day 7 after treatment were comparable between the two groups (0.80 ± 1.17 vs. 0.80 ± 1.20, P = 0.2177). The majority of the participants in both groups had an edema score of 0 at day 7 after treatment [96 (89.72%) vs. 99 (91.67%), P = 0.3705]. Adverse events (AEs) occurred in 9 patients (8.4%) in the study group and 3 patients (2.7%) in the control group. In addition, 5 AEs in the study group and 1 AE in the control group were possibly in association with the study drug.
Compared with the administration of diosmin oral tablets alone, the addition of Jiuhua hemorrhoid suppository to the tablets demonstrates enhanced efficacy in addressing hemorrhoid bleeding, with satisfactory patient adherence and acceptable safety.
Hemorrhoidal disease (HD) is the most prevalent benign anorectal disorder worldwide, with an estimated prevalence between 4.4% and 55.0% among adults of both sexes equally [1-3]. Most guidelines recommend conservative or minimally invasive interventions in the management of early-stage HD and reserve hemorrhoidectomy for grades III and IV HD [4, 5].
Bleeding is one of the most frequent complaints associated with HD, arising from erosion or trauma to the underlying blood vessels [6]. Diosmin is a plant-derived flavonoid with known therapeutic anti-cancer, anti-bacteria, anti-cardiovascular effects, as well as the function of liver protection and neuro-protection. It has been applied for the treatment of hemorrhoids, varicose veins, venous insufficiency, leg ulcers, and various circulatory diseases for almost a century [7]. Clinical trials have been conducted with the use of different flavonoids including diosmin, troxerutin, quercetin, rutin, and hesperidin, with satisfactory outcomes such as reduced anal pain and alleviated bleeding, pruritus, anal leakage, and tenesmus achieved [8-10]. A previous meta-analysis study suggested the application of flavonoids for the treatment of hemorrhoids under the condition of it being employed as a supplementary treatment, but no evidence had yet revealed the effectiveness of the drug alone on hemorrhoid treatment [11]. Although oral administration might slow down the treatment effect, giving the drug via the rectal route has been proven beneficial in circumventing undesirable hepatic and gastrointestinal side effects [12]. Hence, topical agents including rectal foams, gels, ointments, and suppositories have been produced to cater to different application needs and have demonstrated notable success. With the dynamic structure of the anal canal, the challenge of prolonged exposure arises, a concern can be addressed by employing thicker gels or suppositories. However, it is noteworthy that only a scarcity of studies have focused on suppositories (Supplementary Table S1).
Numerous traditional Chinese medicine (TCM) suppositories designed for the treatment of hemorrhoids have been introduced to the markets in China. However, only a limited number of reproducible studies have demonstrated clinical evidence supporting their efficacy [13]. Jiuhua hemorrhoid suppository is one of the TCM suppositories with seven main ingredients (Supplementary Table S2). According to the compatibility theory for formula in TCM, Dahuang (Rhei Radix et Rhizoma) serves as the “monarch drug”, Zhebeimu (Fritillariae Thunbergii Bulbus) is the “minister drug”, and the other five, including Cebaiye (Platycladi Cacumen), Bingpian (Borneolum), Houpo (Magnoliae Officinalis Cortex), Baiji (Bletillae Rhizoma), and Zicao (Arnebiae Radix), serve as the “assistant drugs”. While Jiuhua hemorrhoids suppository has been utilized for decades in addressing HD, there are only small-scale studies reporting its efficacy in Chinese patients [14-16], and randomized controlled trials (RCTs) with sufficient statistical power are particularly lacking.
The objective of this study was to compare the efficacy and safety of combining diosmin with Jiuhua hemorrhoid suppository versus diosmin tablets alone for the treatment of hemorrhoid bleeding.
The Jiuhua hemorrhoid suppository study was a RCT conducted in 10 medical centers in China from April 1, 2019 to June 30, 2020. Patients with hemorrhoids who were planning for conservative treatment were enrolled. The study protocol, conducted in accordance with the principles of the Declaration of Helsinki, was registered on the website (http://www.chictr.org.cn/index.aspx) (ChiCTR1800019613) as well. It was also approved by the Ethics Committee of the Affiliated Hospital of Hunan Academy of Chinese Medicine ([201906]14). All patients provided written informed consent before being enrolled.
The diagnosis of hemorrhoids was established following the criteria outlined in the Clinical Practice Guidelines for the Management of Hemorrhoids by the American Society of Colon and Rectal Surgeons [17]. Hemorrhoids were classified as internal, external, or mixed, with internal hemorrhoids further stratified into stages I to IV based on the severity of the condition.
TCM syndromes were diagnosed and classified into four dialectical sub-types according to the Chinese Guidelines for the Clinical Diagnosis and Treatment of Hemorrhoids (2006 edition) [18]. These sub-types include the syndrome of wind damaging the intestinal vessel, syndrome of dampness-heat pouring downward, syndrome of Qi-stagnation and blood stasis, and syndrome of spleen deficiency with sunken Qi.
(i) Patients were aged between 18 and 75 years old; (ii) patients were diagnosed with HD; (iii) patients’ internal hemorrhoids were in stage I/II, or those in stage III/IV but refused surgical treatment; (iv) patients were willing to provide written informed consent before participation in this study. There were no restrictions on the inclusion of specific types of hemorrhoids (internal, external, or mixed) or the related TCM syndromes.
(i) Patients accompanying other diseases: (a) anal and rectal lesions, such as anal fissure, anal fistula, perianal abscess; (b) acute gastrointestinal infections; (c) history of severe gastrointestinal diseases, e.g. Crohn’s disease, ulcerative colitis, peptic ulcer, and peptic haemorrhage; (d) gastrointestinal tumours, pyloric stenosis, and gastric bypass; (e) serious abnormalities of liver and kidney function, with any of the indicators of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum creatinine reaching or exceeding three times the upper limit of normal values; (f) patients with uncontrollable hypertension or other serious systemic diseases that were judged by the investigator to be unsuitable for enrolment; (ii) drug-related: (a) patients having a known history of allergy to the drugs involved in this study; (b) patients taking antiplatelet or anticoagulant drugs; (c) patients with alcohol or drug dependence; (d) patients who have received any haemorrhoid-related treatment within one month prior to enrolment; (iii) patients with symptoms of infectious diseases, epilepsy, pregnant and breastfeeding women, and mental disorders; (iv) participation in other clinical trials within three months.
(i) Cases that did not meet the inclusion criteria but were mistakenly included; (ii) cases that met the inclusion criteria but had not used the drug after inclusion; (iii) cases that were allergic to the drug of interest during the trial; (iv) subjects with poor adherence; (v) occurrence of severe adverse event (AE); (vi) occurrence of serious complications or special physiological changes that make continuation of the trial inadvisable; (vii) cases not in accordance with the provisions of the drug were off cases, statistical analysis should be combined with the actual situation, if adverse reactions occur, they should be counted according to the adverse reactions, and those who had completed 1/2 of the treatment should be counted in terms of therapeutic efficacy.
Patients were divided into two groups using a simple randomization approach. Statistical analysis system (SAS) software (version 9.4, SAS Institute Inc., Cary, North Carolina) was used to generate random numbers, and each number was assigned to one of the two groups with equal probability. Upon meeting the eligibility criteria, patients were assigned treatment using sequentially numbered, opaque, and sealed envelopes, with the treatment allocation in a ratio of 1 : 1. This study was not blinded due to the employment of different drug administration approach.
In the study group, patients orally administered diosmin tablets (Nanjing Zhengda Tianqing Pharmaceutical Co., Ltd., China) at a dosage of 0.9 g, once a day with lunch, and concurrently used Jiuhua hemorrhoid suppository (2.1 g, Jiuhua Pharmaceutical Co., Ltd., China) once a day after defecation or at bedtime after rinsing the anus with warm water (one suppository each time). In the control group, patients orally received diosmin tablets alone at a dosage of 0.9 g once a day.
The efficacy was evaluated at (7 ± 2) days after treatment [10, 19].
The primary endpoint for this study was the relief of hemorrhoid bleeding, described as “very effective” “effective” and “ineffective”. Specifically, “very effective” was defined as patients stopped bleeding within 24 − 48 h after treatment, no bleeding in the subsequent three stools, and sustained absence of bleeding for 7 d. “Effective” was defined as cases with bleeding cessation occurring more than 48 h after medication, accompanied by a significant reduction in bleeding volume (a decrease of at least 2 scores or from 1 to 0 score). Cases were categorized as “ineffective” if their bleeding volume was not significantly reduced.
Bleeding scores ranged from 0 to 3, with 0 denoting the absence of bleeding symptoms during defecation, 1 for the presence of blood in the stool, 2 for blood dripping down after defecation, and 3 for severe jet bleeding during defecation.
The secondary endpoints included a decrease in hemorrhoid pain scores and edema scores. Hemorrhoid pain scores were evaluated using the visual analogue scale (VAS), where 0 meant no pain, 1 − 3 mild pain, 4 − 6 moderate pain, and 7 − 10 severe pain. The relief scores of hemorrhoid edema ranged from 0 to 3, with 0 denoting no edema, 1 for the edema area reduced by no less than 2/3, 2 for the edema area reduced by no less than 1/2 and no more than 2/3, and 3 for the edema area reduced by no more than 1/2 of its original size.
Subgroup analyses were conducted to compare the relief of bleeding according to the location, stage, and TCM dialectical sub-types of hemorrhoids.
Patients were followed (14 ± 2) days after treatment for the observation of AEs. The vital sign, complete blood count, liver function, renal function, urine routine, fecal routine, and blood human chorionic gonadotropin were tested, and electrocardiogram was conducted at each follow-up visit. All AEs were recorded. Medication adherence was calculated as the ratio of the actual dose of the drugs to the prescribed dose, and adherence of 80% − 120% was considered satisfactory.
According to previous study [20], the relief rate of hemorrhoid bleeding after treatment for one week was estimated to be 85% in the study group and 66% in the control group. With significance level α = 0.05 and power of the test = 0.8, each group required 80 cases. Taking into account a lost-to-follow-up rate of 20%, 100 cases were enrolled in both groups.
The full analysis set (FAS) and per-protocol set (PPS) were used to analyze the primary endpoint. The FAS included randomized patients who had received study drugs at least once and underwent at least one follow-up assessment for the determination of the efficacy and safety of the drugs. The PPS was a subset of the FAS and excluded patients who took prohibited drugs as per the protocol and those who significantly deviated the prescribed usage and dosage of the study drugs. The secondary endpoints and subgroup analyses were conducted in the PPS. The safety set (SS) included patients who received study drugs at least once, and were assessed for the safety of the drugs. The statistical analysis did not include missing data.
Continuous variables with normal distribution were presented as means ± standard deviation (SD). Continuous variables with non-normal distribution were presented as medians [interquartile range (IQR)]. Paired t test and the Wilcoxon rank test were used to compare the variables before and after treatment, while the Student’s t test and Wilcoxon rank test were used to compare the variables between the two groups. Categorical and ordinal variables were presented as numbers and percentages. Chi-square or Fisher’s exact test was used for between-group comparison, as appropriate (Fisher’s exact test was used when the expected values in any of the cells of a contingency table were below 5 or below 10 when there was only one degree of freedom). The Wilcoxon rank sum test was used and P < 0.05 was considered statistically significant. Statistical analyses were conducted in SAS (version 9.4).
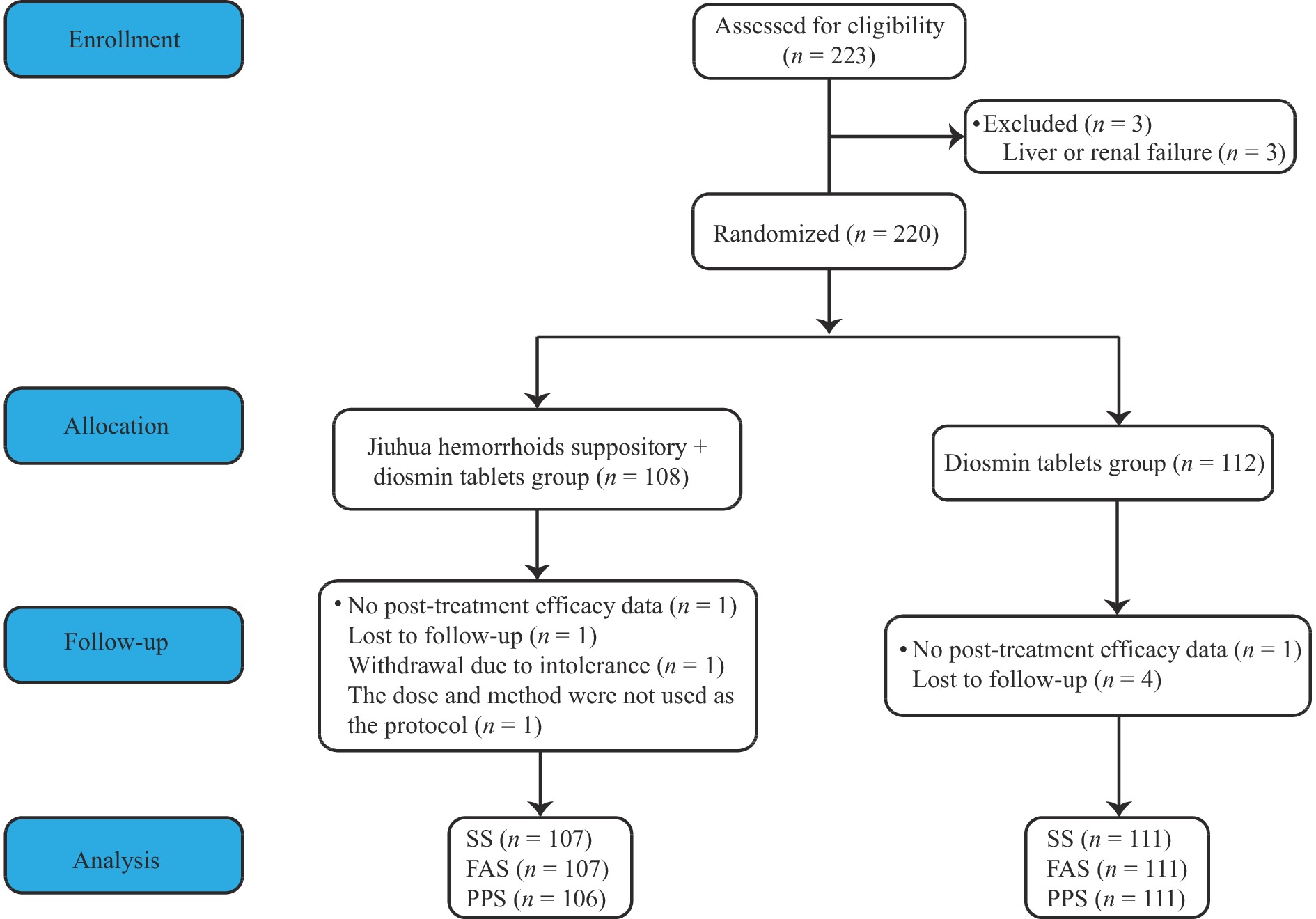
A total of 223 patients were assessed for eligibility to the study, of which 3 were excluded because of liver or renal failure. Thus, a total of 220 were randomized, with 108 patients in the study group and 112 in the control group (Figure 1). There were no statistical differences in age, gender, nationality education level, BMI, baseline hematochezia score, pain VAS score, edema score, types and stages of hemorrhoids, and TCM dialectical sub-type between the two groups (P > 0.05) (Table 1).
| Group | Age (years) | Male [n (%)] | Nationality [n (%)] | Education level [n (%)] | ||||
| Han | Tibetan | Illiteracy | Primary school | Junior high school | ||||
| Study group (n = 107) | 41.9 ± 13.7 | 47 (43.93) | 107 (100.00) | 0 | 1 (0.94) | 6 (5.61) | 23 (21.49) | |
| Control group (n = 111) | 40.8 ± 14.3 | 40 (36.04) | 109 (98.20) | 2 (1.80) | 4 (3.60) | 9 (8.11) | 14 (12.61) | |
| Total (n = 218) | 41.3 ± 14.0 | 87 (39.91) | 216 (99.08) | 2 (0.92) | 5 (2.29) | 15 (6.88) | 37 (16.97) | |
| P value | 0.5675 | 0.2355 | 0.1660 | |||||
| Group | Education level [n (%)] | BMI | |||||
| High school/technical secondary school |
University/ college |
Master | PhD and above | Valid/missing (n) |
Mean ± SD (kg/m2) |
||
| Study group (n = 107) | 18 (16.82) | 31 (28.97) | 27 (25.23) | 1 (0.94) | 56 /51 | 23.0 ± 3.6 | |
| Control group (n = 111) | 22 (19.82) | 42 (37.84) | 19 (17.12) | 1 (0.90) | 61/50 | 23.6 ± 4.5 | |
| Total (n = 218) | 40 (18.35) | 73 (33.49) | 46 (21.10) | 2 (0.92) | 117/101 | 23.3 ± 4.1 | |
| P value | 0.5770 | 0.3783 | |||||
| Group | Hematochezia score [n (%)] | Pain VAS score | Edema score [n (%)] | ||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||
| Study group (n = 107) | 1 (0.94) | 34 (31.78) | 60 (56.07) | 12 (11.21) | 1.9 ± 1.87 | 78 (72.90) | 16 (14.95) | 11 (10.28) | 2 (1.87) |
| Control group (n = 111) | 1 (0.90) | 43 (38.74) | 60 (54.05) | 7 (6.31) | 1.8 ± 1.88 | 87 (78.38) | 11 (9.91) | 11 (9.91) | 2 (1.80) |
| Total (n = 218) | 2 (0.92) | 77 (35.32) | 120 (55.05) | 19 (8.71) | 1.9 ± 1.87 | 165 (75.69) | 27 (12.39) | 22 (10.09) | 4 (1.83) |
| P value | 0.1783 | 0.6019 | 0.0426 | ||||||
| Group | Type of hemorrhoids [n (%)] | Stage of hemorrhoids [n (%)] | |||||
| Internal hemorrhoids | Mixed hemorrhoids | I | II | III | Unknown | ||
| Study group (n = 107) | 42 (39.25) | 65 (60.75) | 20 (18.69) | 48 (44.86) | 6 (5.61) | 33 (30.84) | |
| Control group (n = 111) | 56 (50.45) | 55 (49.55) | 35 (31.53) | 52 (46.85) | 4 (3.60) | 20 (18.02) | |
| Total (n = 218) | 98 (44.95) | 120 (55.05) | 55 (25.23) | 100 (45.87) | 10 (4.59) | 53 (24.31) | |
| P value | 0.0976 | 0.0905 | |||||
| Group | TCM dialectical sub-type [n (%)] | ||||
| Syndrome of wind damaging the intestinal vessel |
Syndrome of dampness-heat pouring downward |
Syndrome of Qi stagnation and blood stasis |
Syndrome of spleen deficiency with sunken Qi |
Unknown | |
| Study group (n = 107)* | 27 (25.23) | 59 (55.14) | 2 (1.87) | 10 (9.35) | 10 (9.35) |
| Control group (n = 111) | 38 (34.23) | 54 (48.65) | 3 (2.70) | 9 (8.11) | 7 (6.31) |
| Total (n = 218)* | 65 (29.82) | 113 (51.83) | 5 (2.29) | 19 (8.72) | 17 (7.80) |
| P value | 0.2404 | ||||
| BMI, body mass index. *One participant in study group has both syndrome of Qi stagnation and blood stasis and syndrome of spleen deficiency with sunken Qi. | |||||
In terms of the treatment efficacy against hemorrhoid bleeding, the proportion of very effective and effective cases in the study group was significantly higher in comparison with that in the control group in FAS [106 (99.06%) vs. 91 (81.98%), P < 0.0001]. The results of PPS [105 (99.06%) vs. 91 (81.98%), P < 0.0001] were comparable to those of FAS (Table 2 and 3).
| Group | Very effective | Effective | Ineffective |
| Study group (n = 107) | 49 (45.79) | 57 (53.27) | 1 (0.94) |
| Control group (n = 111) | 18 (16.22) | 73 (65.76) | 20 (18.02) |
| P value | < 0.0001* | ||
| *P value for effectiveness rate; effectiveness rate = very effective + effective. | |||
| Group | Very effective | Effective | Ineffective |
| Study group (n = 106) | 49 (46.23) | 56 (52.83) | 1 (0.94) |
| Control group (n = 111) | 18 (16.22) | 73 (65.76) | 20 (18.02) |
| P value | < 0.0001* | ||
| *P value for effectiveness rate; effectiveness rate = very effective + effective. | |||
The pain VAS scores at day 7 after treatment were similar between the two groups (0.80 ± 1.17 vs. 0.80 ± 1.20, P = 0.2177). The majority of participants in both groups had an edema score of 0 at day 7 after treatment [96 (89.72%) vs. 99 (91.67%), P = 0.3705] (Table 4 and 5). Numerically higher proportions of very effective and effective cases in the study group were observed in the subgroups of internal hemorrhoids, mixed hemorrhoids, stage I and II hemorrhoids, and the syndrome of wind damaging the intestinal vessel (Figure 2).
| Group | Pain VAS score at baseline |
Pain VAS score after treatment (day 7) |
P valuea |
| Study group (n = 107) | 1.9 ± 1.87 | 0.8 ± 1.17 | < 0.0001 |
| Control group (n = 111) | 1.8 ± 1.88 | 0.8 ± 1.20* | < 0.0001 |
| P value | 0.6019 | 0.2177 | |
| a P value compared with baseline. *Data of 3 cases are missing. | |||
| Group | Edema score at baseline | Edema score after treatment (day 7) | P valuea | |||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |||
| Study group (n = 107) | 78 (72.90) | 16 (14.95) | 11 (10.28) | 2 (1.87) | 96 (89.72) | 11 (10.28) | 0 | 0 | < 0.0001 | |
| Control group (n = 111) | 87 (78.38) | 11 (9.91) | 11 (9.91) | 2 (1.80) | 99 (91.67) | 9 (8.33) | 0 | 0 | < 0.0001 | |
| P value | 0.4026 | 0.3705 | ||||||||
| a P value compared with baseline. Data of 3 cases in control group are missing at day 7 after treatment. | ||||||||||
As shown in Table 6, 9 AEs occurred in 9 patients (8.4%) in the study group, and 4 AEs occurred in 3 patients (2.7%) in the control group. There was 1 severe case of coughing in the study group and 1 severe case of increased urination case at night in the control group, while all other AEs were considered mild. Among these, 5 AEs in the study group and 1 in the control group were considered probably related to the study drugs.
| AEs | Study group (n = 107) | Control group (n = 111) | Total (n = 218) |
| Elevated WBC | 0 | 1 | 1 |
| Increased bowel movements, active bowel sounds | 1 | 0 | 1 |
| Nausea with bloating | 1 | 0 | 1 |
| Bloating (flatulence) | 1 | 0 | 1 |
| Cold | 0 | 1 | 1 |
| Anal pain and bulging | 1 | 0 | 1 |
| Cough | 1* | 0 | 1 |
| Urinary system infection | 1 | 0 | 1 |
| Itchy skin | 1 | 0 | 1 |
| Dizziness | 1 | 0 | 1 |
| Reduced hemoglobin | 1 | 0 | 1 |
| Increased urination at night | 0 | 1* | 1 |
| Elevated neutrophils | 0 | 1 | 1 |
| Total | 9 | 4 | 13 |
| WBC, white blood cell. * represtents severe AE. | |||
In the study group, satisfactory adherence to the prescribed regimen was reported, with 96.3% of cases adhering to Jiuhua hemorrhoid suppository and 95.4% to diosmin tablets [1 case (0.9%) lacked adherence data]. Similarly, in the control group, satisfactory adherence was reported in 96.4% of the cases [3 cases (2.7%) lacked adherence data].
To the best of our knowledge, this is the first multi-center, randomized, and controlled trial reporting the benefits of the Jiuhua hemorrhoid suppository for the conservative treatment of HD. Although no significant differences were found between the two groups in the relief scores of pain and edema, adding Jiuhua hemorrhoid suppository to the treatment regimen for hemorrhoids was more effective than the application of diosmin tablets alone, with an acceptable safety profile.
Diosmin and other flavonoids, such as troxerutin, quercetin, rutin, and hesperidin, are recommended for HD treatment due to their venoprotective effects, aiming to prevent venous damage and bleeding [8]. Clinical trials with approval have reported a reduction in anal pain and bleeding in more than 75% of patients after 7 days of diosmin treatment, even in both early and late stages of HD [9, 21]. However, it is essential to note that the pathogenesis and clinical manifestations of HD are complex. Consequently, treatment is not always straightforward, and a prescription of oral flavonoids may not be sufficient. In such cases, the combination of oral treatment, including flavonoids, with topical treatment creates favorable conditions for a smoother recovery.
The rectal route for drug administration proves advantageous in circumventing undesirable hepatic and gastrointestinal side effects [12]. In addition, the activity of the drugs will not be affected by gastrointestinal pH or enzymes. Furthermore, giving the drug through rectal routes could lead to smoother drug release, longer action time, and faster onset of action than oral tablets does. On top of all that, suppositories are convenient to carry, store, and use. Thus, in many clinical trials, rectal formulations often integrate already approved drugs into conventional rectal forms [22]. However, the current perspectives of medical society on topical treatment is not favorable for HD patients. Even though short-term treatment has been reported successful, this result has not shared by well-designed and robust studies [1]. What’s worse, global concerns have been raised over reports on local reactions or sensitization under the circumstance [13]. In our study, the number of patients who received additional Jiuhua hemorrhoid suppository and consequently showed efficacy on relieving hemorrhoid bleeding was significantly higher in comparison with that of patients who administered the diosmin tablets alone. Subgroup analysis also reported beneficial results for the addition of Jiuhua hemorrhoid suppository. The consistent findings indicated that Jiuhua hemorrhoid suppository was suitable for patients with various types of hemorrhoids, regardless of the location, stage, and TCM dialectical sub-type. However, it is crucial to interpret the results of subgroup analysis cautiously due to the relatively small sample size. Combining a systemic drug with a local agent could tackle the problem from two fronts at once, potentially achieving ideal clinical effects and accelerating the onset of treatment effects.
TCM possesses a long history of effectively treating HD, with commonly reported herbs encompassing Diyu (Sanguisorbae Radix), Dihuang (Rehmanniae Radix), Huaijiao (Sophorae Fructus), Danggui (Angelicae Sinensis Radix), Huangqin (Scutellariae Radix), and Cebaiye (Platycladi Cacumen) [13]. However, the dosage and composition of the treatment are often not reported, limiting the study’s reproducibility, thus the popular saying that TCM herbs are not very useful as local treatment for stopping bleeding because of hemorrhoids [13]. In this study, we reported the role of Jiuhua hemorrhoid suppository, a compound in TCM consisting of seven plant-derived materials with a rich history in TCM application, for the treatment of hemorrhoid bleeding. According to the TCM theory, the “monarch drug” Dahuang (Rhei Radix et Rhizoma) is rigorously compatible with the “minister drug” Zhebeimu (Fritillariae Thunbergii Bulbus) and the other “assistant drugs” including Cebaiye (Platycladi Cacumen), Bingpian (Borneolum), Houpo (Magnoliae Officinalis Cortex), Baiji (Bletillae Rhizoma), and Zicao (Arnebiae Radix) [15]. The seven components of Jiuhua hemorrhoid suppository have proven effects that can be applied in treating hemorrhoids at different pathogenetic levels (Supplementary Figure S1). The pharmacological analysis also supported the efficacy of Jiuhua hemorrhoid suppository. Dahuang (Rhei Radix et Rhizoma) is bitter and cold, with efficacy on clearing heat, purging fire, promoting blood circulation, and removing blood stasis. Dahuang (Rhei Radix et Rhizoma) has multiple pharmacological effects, including anti-tumor, anti-inflammatory, and inhibition of fibrosis. The active ingredients, including α-catechin, rhein, and emodin, are effective in eliminating hemostasis and hemorrhoids [23, 24]. Rhaponticin and its aglycone form, rhapontigenin, inhibit the activation of cyclooxygenase, lipoxygenase, and hyaluronoglucosaminidase, thereby modulating various pro-inflammatory responses and exerting anti-inflammatory effects [25]. Anthraquinones derivatives, essential active components of Dahuang (Rhei Radix et Rhizoma), play a crucial role in promoting thrombopoiesis, considerably increasing fibrinogen, shortening the clotting time, as well as stopping bleeding [26]. Zhebeimu (Fritillariae Thunbergii Bulbus) is bitter and cold in nature, contributing to its ability to clear away heat, disperse knots, soften dry feces, and reduce the resting pressure of the anal canal [27]. Zhebeimu (Fritillariae Thunbergii Bulbus) has anti-inflammatory and anti-oxidant features with the capacity to suppress pain. The chemical compound Peimine (A1) can block the Kv1.3 ion channel, a mechanism often associated with anti-inflammatory effects and the inhibition of immune responses induced by human T lymphocytes. Peimine (A1) could block the Nav1.7 ion channel and suppress pain as well. In addition, the total alkaloids and polysaccharides extracted from Zhebeimu (Fritillariae Thunbergii Bulbus) revealed a strong anti-oxidative capacity in vitro [27]. Baiji (Bletillae Rhizoma) is bitter, sweet, astringent, and slightly cold in nature, with the ability to stop bleeding and reduce body swelling. Baiji (Bletillae Rhizoma) polysaccharides participate in multiple hemostasis processes, including facilitating platelet adherence to the subendothelial matrix to block vessels and promoting the formation of fibrin clots through activating coagulation factors and enhancing thromboxane-A2 synthesis [28]. Cebaiye (Platycladi Cacumen) is bitter, sweet, astringent, and slightly cold, which could cool blood and stop bleeding. Extracts and compounds of Cebaiye (Platycladi Cacumen) leaves can reduce the mPGE 2 concentration and inhibit the production of interleukin-6 (IL-6) and nitric oxide (NO), with anti-inflammatory, anti-oxidant, and anti-fibrotic features. The essential oil of Cebaiye (Platycladi Cacumen) leaves is considered to have anti-oxidant effects by improving the cellular anti-oxidative defense system, inhibiting glutathione and malondialdehyde production, as well as preserving the activity of superoxide dismutase. Zicao (Arnebiae Radix) is bitter, astringent, and cool. It could be applied for clearing away heat, cooling blood, relaxing bowels, and promoting the healing of hemorrhoid mucosa [29]. Naphthoquinone compounds of the Zicao (Arnebiae Radix) suppress tumor necrosis factor α (TNF-ᾳ) and nuclear factor kappa-B (NF-κB), the synthesis of leukotriene and prostaglandin, and the production of IL-6, showing anti-inflammatory and anti-oxidative effects as well. Besides, the Zicao (Arnebiae Radix) ointments are notable for the wound-healing properties, which might be related to the anti-microbial effects they exert in human body [29]. Houpo (Magnoliae Officinalis Cortex) is bitter, acrid, and warm, which could promote Qi circulation and remove dampness. Lignans, major significant bioactive components isolated from the bark of Houpo (Magnoliae Officinalis Cortex), target multiple signaling pathways, including NF-κB and signal transducer and activator of transcription 3 (STAT3), which are associated with metabolism and inflammation. Down-regulating and blocking NF-κB activation is possibly one of the anti-inflammatory mechanisms [30]. Bingpian (Borneolum) is acrid, bitter, and slightly cold, with the properties of clearing away heat, relieving pain and body swelling [31]. Bingpian (Borneolum), the processed item from the resin Cinnamomum Camphora (L.) Presl, can improve energy metabolism and enhance the activity of anti-oxidant enzymes in tissues, thus reducing leukocyte infiltration and inflammatory response [31-33]. Therefore, Jiuhua hemorrhoid suppository can reduce body swelling, resolve blood stasis, stop bleeding, clear heat, and relieve pain.
Regarding safety, 10 AEs occurred in 9 patients (8.4%) in the study group and 4 AEs in 3 patients (2.7%) in the control group, of which 5 AEs in the study group and 1 AE in the control group were considered in association with the study drugs. Increased bowel movements and bloating might be rare side effects of the Dahuang (Rhei Radix et Rhizoma) component [24]. Of all AEs in the study group, 9 cases presented only mild symptoms, but 1 case had severe cough, suggesting that Jiuhua hemorrhoid suppository was acceptable for the safety of patients with stage I or II hemorrhoids, as well as stage III and IV patients who refused surgical treatment.
There are some limitations to the study. Firstly, the treatment and follow-up periods were relatively brief, given that patients with grade I and II hemorrhoid hemorrhage typically could be cured within seven days. Similar studies and protocols advocate for evaluating effectiveness seven days after treatment [19, 34]. Secondly, a placebo suppository was not used due to the following considerations: with no effective therapeutic ingredients, a placebo suppository may increase bleeding and pain in patients; the study suppository has a special smell, which is hard to be replaced by a placebo suppository with similar smell but without pharmaceutical ingredients. Thus, the study was not blinded, which might lead to assessment biases.
In conclusion, compared with oral diosmin tablets alone, combining Jiuhua hemorrhoid suppository with the treatment regimen for hemorrhoid bleeding showed increased effectiveness. Acceptable safety and adherence data support its use as an additional topical treatment for hemorrhoids.
Scientific Research Subject of Shanghai Municipal Health and Family Planning Commission (2017Y0233).
Competing Interests: The authors declare no conflict of interest
| [1] |
SALGUEIRO P, CAETANO AC, OLIVEIRA AM, et al. Portuguese society of gastroenterology consensus on the diagnosis and management of hemorrhoidal disease. GE Portuguese Journal of Gastroenterology, 2020, 27(2): 90–102. doi: 10.1159/000502260
|
| [2] |
SANDLER RS, PEERY AF. Rethinking what we know about hemorrhoids. Clinical Gastroenterology and Hepatology, 2019, 17(1): 8–15. doi: 10.1016/j.cgh.2018.03.020
|
| [3] |
SHEIKH P, RÉGNIER C, GORON F, et al. The prevalence, characteristics and treatment of hemorrhoidal disease: results of an international web-based survey. Journal of Comparative Effectiveness Research, 2020, 9(17): 1219–1232. doi: 10.2217/cer-2020-0159
|
| [4] |
SOBRADO CW, DE ALMEIDA OBREGON C, SOBRADO LF, et al. The novel BPRST classification for hemorrhoidal disease: a cohort study and an algorithm for treatment. Annals of Medicine and Surgery, 2021, 61: 97–100. doi: 10.1016/j.amsu.2020.12.019
|
| [5] |
YAMANA T. Japanese practice guidelines for anal disorders I. hemorrhoids. Journal of the Anus, Rectum and Colon, 2017, 1(3): 89–99. doi: 10.23922/jarc.2017-018
|
| [6] |
GALLO G, MISTRANGELO M, PASSERA R, et al. Efficacy of mesoglycan in pain control after excisional hemorrhoidectomy: a pilot comparative prospective multicenter study. Gastroenterology Research and Practice, 2018, 2018: 6423895. doi: 10.1155/2018/6423895
|
| [7] |
HUWAIT E, MOBASHIR M. Potential and therapeutic roles of diosmin in human diseases. Biomedicines, 2022, 10(5): 1076. doi: 10.3390/biomedicines10051076
|
| [8] |
CORSALE I, CARRIERI P, MARTELLUCCI J, et al. Flavonoid mixture (diosmin, troxerutin, rutin, hesperidin, quercetin) in the treatment of I-III degree hemorroidal disease: a double-blind multicenter prospective comparative study. International Journal of Colorectal Disease, 2018, 33(11): 1595–1600. doi: 10.1007/s00384-018-3102-y
|
| [9] |
SHELYGIN Y, KRIVOKAPIC Z, FROLOV SA, et al. Clinical acceptability study of micronized purified flavonoid fraction 1000 mg tablets versus 500 mg tablets in patients suffering acute hemorrhoidal disease. Current Medical Research and Opinion, 2016, 32(11): 1821–1826. doi: 10.1080/03007995.2016.1211520
|
| [10] |
SHEIKH P, LOHSIRIWAT V, SHELYGIN Y. Micronized purified flavonoid fraction in hemorrhoid disease: a systematic review and meta-analysis. Advances in Therapy, 2020, 37(6): 2792–2812. doi: 10.1007/s12325-020-01353-7
|
| [11] |
BASHANKAEV BN, WEXNER SD, ARKHAROV AV. Common sense of diosmin administration in combined treatment of hemorrhoids. Khirurgiia, 2018(8.Vyp.2): 83–89. doi: 10.17116/hirurgia201808283
|
| [12] |
BELNIAK P, ŚWIĄDER K, SZUMIŁO M, et al. Comparison of physicochemical properties of suppositories containing starch hydrolysates. Saudi Pharmaceutical Journal, 2017, 25(3): 365–369. doi: 10.1016/j.jsps.2016.09.004
|
| [13] |
GALLO G, MARTELLUCCI J, STURIALE A, et al. Consensus statement of the Italian society of colorectal surgery (SICCR): management and treatment of hemorrhoidal disease. Techniques in Coloproctology, 2020, 24(2): 145–164. doi: 10.1007/s10151-020-02149-1
|
| [14] |
DING YS. Efficacy of Jiuhua hemorrhoid suppository in combination with diosmin tablets in the treatment of acute hemorrhoid attacks. Hebei Medical Journal, 2011, 33(24): 3747–3748. doi: 10.3969/j.issn.1002-7386.2011.24.033
|
| [15] |
XU SJ. Clinical observation of Jiuhua hemorrhoid suppository for the treatment of 58 patients with internal hemorrhoids. Journal of Chinese Medicinal Materials, 2003, 26(12): 2.
|
| [16] |
HUANG YB, CAI LL, CHEN GW. Clinical observation of Jiuhua hemorrhoid suppository for the treatment of 60 patients with internal hemorrhoids. China Pratical Medicine, 2009, 4(33): 112–113.
|
| [17] |
DAVIS BR, LEE-KONG SA, MIGALY J, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Hemorrhoids. Diseases of the Colon and Rectum, 2018, 61(3): 284–292. doi: 10.1097/DCR.0000000000001030
|
| [18] |
The Subspecialty Group of Colorectal and Anal Surgery Cmap, Professional Committee of Colorectal and Anal Disease. Guidelines for the clinical diagnosis and treatment of hemorrhoids (2006 edition). Chinese Journal of Gastrointestinal Surgery, 2006, 2006(9): 461–463.
|
| [19] |
GRAVINA AG, PELLEGRINO R, FACCHIANO A, et al. Evaluation of the efficacy and safety of a compound of micronized flavonoids in combination with vitamin C and extracts of Centella asiatica, Vaccinium myrtillus, and Vitis vinifera for the reduction of hemorrhoidal symptoms in patients with grade II and III hemorrhoidal disease: a retrospective real-life study. Frontiers in Pharmacology, 2021, 12: 773320. doi: 10.3389/fphar.2021.773320
|
| [20] |
LAN F, ZHAO G. Treatment efficacy and effect on quality of life in patients of diosmin combined with musk hemorrhoids ointment hemorrhoids. Journal of Liaoning University of Traditional Chinese Medicine, 2016, 18(4): 225–227.
|
| [21] |
ZAGRIADSKIĬ EA, BOGOMAZOV AM, GOLOVKO EB. Conservative treatment of hemorrhoids: results of an observational multicenter study. Advances in Therapy, 2018, 35(11): 1979–1992. doi: 10.1007/s12325-018-0794-x
|
| [22] |
HUA SS. Physiological and pharmaceutical considerations for rectal drug formulations. Frontiers in Pharmacology, 2019, 10: 1196. doi: 10.3389/fphar.2019.01196
|
| [23] |
NI ZH, WU L, CAO KX, et al. Investigation of the pharmacodynamic substances in Dahuang Zhechong pill that inhibit energy metabolism. Journal of Ethnopharmacology, 2020, 251: 112332. doi: 10.1016/j.jep.2019.112332
|
| [24] |
TSANG SW, BIAN ZX. Anti-fibrotic and anti-tumorigenic effects of Rhein, a natural anthraquinone derivative, in mammalian stellate and carcinoma cells. Phytotherapy Research, 2015, 29(3): 407–414. doi: 10.1002/ptr.5266
|
| [25] |
XIANG H, ZUO JX, GUO FY, et al. What we already know about rhubarb: a comprehensive review. Chinese Medicine, 2020, 15: 88. doi: 10.1186/s13020-020-00370-6
|
| [26] |
LIU JT, WU J, WANG HR, et al. Spraying rhubarb powder solution under gastroscope in the treatment of acute non-varicose upper gastrointestinal bleeding: a systematic review and meta-analysis of randomized controlled trials. Complementary Therapies in Medicine, 2020, 52: 102476. doi: 10.1016/j.ctim.2020.102476
|
| [27] |
LI H, HUNG A, LI MD, et al. Fritillariae Thunbergii Bulbus: Traditional uses, phytochemistry, pharmacodynamics, pharmacokinetics and toxicity. International Journal of Molecular Sciences, 2019, 20(7): 1667. doi: 10.3390/ijms20071667
|
| [28] |
XU DL, PAN YC, CHEN JS. Chemical constituents, pharmacologic properties, and clinical applications of Bletilla striata. Frontiers in Pharmacology, 2019, 10: 1168. doi: 10.3389/fphar.2019.01168
|
| [29] |
KUMAR A, SHASHNI S, KUMAR P, et al. Phytochemical constituents, distributions and traditional usages of Arnebia euchroma: a review. Journal of Ethnopharmacology, 2021, 271: 113896. doi: 10.1016/j.jep.2021.113896
|
| [30] |
POIVRE M, DUEZ P. Biological activity and toxicity of the Chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents. Journal of Zhejiang University Science B, 2017, 18(3): 194–214. doi: 10.1631/jzus.B1600299
|
| [31] |
KONG QX, WU ZY, CHU X, et al. Study on the anti-cerebral ischemia effect of borneol and its mechanism. African Journal of Traditional, Complementary, and Alternative Medicines, 2014, 11(1): 161–164. doi: 10.4314/ajtcam.v11i1.25
|
| [32] |
LIN S, ZANG MC. Effectiveness of Mayinglong musk hemorrhoid ointment on wound healing and complications after internal hemorrhoid ligation and external hemorrhoidectomy. Evidence-Based Complementary and Alternative Medicine, 2022, 2022: 5630487. doi: 10.1155/2022/5630487
|
| [33] |
SHA Q, CHENG M, ZHOU F, et al. Effects of Huhuang Burn Liniment on wound healing and changes in IL-10 and MMP-9 levels in patients with mixed hemorrhoids. American Journal of Translational Research, 2022, 14(10): 7434–7442.
|
| [34] |
SHI SY, ZHOU Q, HE ZQ, et al. Traditional Chinese medicine (Liangxue Dihuang Decoction) for hemorrhoid hemorrhage: study protocol clinical trial (SPIRIT compliant). Medicine, 2020, 99(16): e19720. doi: 10.1097/MD.0000000000019720
|
| Group | Age (years) | Male [n (%)] | Nationality [n (%)] | Education level [n (%)] | ||||
| Han | Tibetan | Illiteracy | Primary school | Junior high school | ||||
| Study group (n = 107) | 41.9 ± 13.7 | 47 (43.93) | 107 (100.00) | 0 | 1 (0.94) | 6 (5.61) | 23 (21.49) | |
| Control group (n = 111) | 40.8 ± 14.3 | 40 (36.04) | 109 (98.20) | 2 (1.80) | 4 (3.60) | 9 (8.11) | 14 (12.61) | |
| Total (n = 218) | 41.3 ± 14.0 | 87 (39.91) | 216 (99.08) | 2 (0.92) | 5 (2.29) | 15 (6.88) | 37 (16.97) | |
| P value | 0.5675 | 0.2355 | 0.1660 | |||||
| Group | Education level [n (%)] | BMI | |||||
| High school/technical secondary school |
University/ college |
Master | PhD and above | Valid/missing (n) |
Mean ± SD (kg/m2) |
||
| Study group (n = 107) | 18 (16.82) | 31 (28.97) | 27 (25.23) | 1 (0.94) | 56 /51 | 23.0 ± 3.6 | |
| Control group (n = 111) | 22 (19.82) | 42 (37.84) | 19 (17.12) | 1 (0.90) | 61/50 | 23.6 ± 4.5 | |
| Total (n = 218) | 40 (18.35) | 73 (33.49) | 46 (21.10) | 2 (0.92) | 117/101 | 23.3 ± 4.1 | |
| P value | 0.5770 | 0.3783 | |||||
| Group | Hematochezia score [n (%)] | Pain VAS score | Edema score [n (%)] | ||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||
| Study group (n = 107) | 1 (0.94) | 34 (31.78) | 60 (56.07) | 12 (11.21) | 1.9 ± 1.87 | 78 (72.90) | 16 (14.95) | 11 (10.28) | 2 (1.87) |
| Control group (n = 111) | 1 (0.90) | 43 (38.74) | 60 (54.05) | 7 (6.31) | 1.8 ± 1.88 | 87 (78.38) | 11 (9.91) | 11 (9.91) | 2 (1.80) |
| Total (n = 218) | 2 (0.92) | 77 (35.32) | 120 (55.05) | 19 (8.71) | 1.9 ± 1.87 | 165 (75.69) | 27 (12.39) | 22 (10.09) | 4 (1.83) |
| P value | 0.1783 | 0.6019 | 0.0426 | ||||||
| Group | Type of hemorrhoids [n (%)] | Stage of hemorrhoids [n (%)] | |||||
| Internal hemorrhoids | Mixed hemorrhoids | I | II | III | Unknown | ||
| Study group (n = 107) | 42 (39.25) | 65 (60.75) | 20 (18.69) | 48 (44.86) | 6 (5.61) | 33 (30.84) | |
| Control group (n = 111) | 56 (50.45) | 55 (49.55) | 35 (31.53) | 52 (46.85) | 4 (3.60) | 20 (18.02) | |
| Total (n = 218) | 98 (44.95) | 120 (55.05) | 55 (25.23) | 100 (45.87) | 10 (4.59) | 53 (24.31) | |
| P value | 0.0976 | 0.0905 | |||||
| Group | TCM dialectical sub-type [n (%)] | ||||
| Syndrome of wind damaging the intestinal vessel |
Syndrome of dampness-heat pouring downward |
Syndrome of Qi stagnation and blood stasis |
Syndrome of spleen deficiency with sunken Qi |
Unknown | |
| Study group (n = 107)* | 27 (25.23) | 59 (55.14) | 2 (1.87) | 10 (9.35) | 10 (9.35) |
| Control group (n = 111) | 38 (34.23) | 54 (48.65) | 3 (2.70) | 9 (8.11) | 7 (6.31) |
| Total (n = 218)* | 65 (29.82) | 113 (51.83) | 5 (2.29) | 19 (8.72) | 17 (7.80) |
| P value | 0.2404 | ||||
| BMI, body mass index. *One participant in study group has both syndrome of Qi stagnation and blood stasis and syndrome of spleen deficiency with sunken Qi. | |||||
| Group | Very effective | Effective | Ineffective |
| Study group (n = 107) | 49 (45.79) | 57 (53.27) | 1 (0.94) |
| Control group (n = 111) | 18 (16.22) | 73 (65.76) | 20 (18.02) |
| P value | < 0.0001* | ||
| *P value for effectiveness rate; effectiveness rate = very effective + effective. | |||
| Group | Very effective | Effective | Ineffective |
| Study group (n = 106) | 49 (46.23) | 56 (52.83) | 1 (0.94) |
| Control group (n = 111) | 18 (16.22) | 73 (65.76) | 20 (18.02) |
| P value | < 0.0001* | ||
| *P value for effectiveness rate; effectiveness rate = very effective + effective. | |||
| Group | Pain VAS score at baseline |
Pain VAS score after treatment (day 7) |
P valuea |
| Study group (n = 107) | 1.9 ± 1.87 | 0.8 ± 1.17 | < 0.0001 |
| Control group (n = 111) | 1.8 ± 1.88 | 0.8 ± 1.20* | < 0.0001 |
| P value | 0.6019 | 0.2177 | |
| a P value compared with baseline. *Data of 3 cases are missing. | |||
| Group | Edema score at baseline | Edema score after treatment (day 7) | P valuea | |||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |||
| Study group (n = 107) | 78 (72.90) | 16 (14.95) | 11 (10.28) | 2 (1.87) | 96 (89.72) | 11 (10.28) | 0 | 0 | < 0.0001 | |
| Control group (n = 111) | 87 (78.38) | 11 (9.91) | 11 (9.91) | 2 (1.80) | 99 (91.67) | 9 (8.33) | 0 | 0 | < 0.0001 | |
| P value | 0.4026 | 0.3705 | ||||||||
| a P value compared with baseline. Data of 3 cases in control group are missing at day 7 after treatment. | ||||||||||
| AEs | Study group (n = 107) | Control group (n = 111) | Total (n = 218) |
| Elevated WBC | 0 | 1 | 1 |
| Increased bowel movements, active bowel sounds | 1 | 0 | 1 |
| Nausea with bloating | 1 | 0 | 1 |
| Bloating (flatulence) | 1 | 0 | 1 |
| Cold | 0 | 1 | 1 |
| Anal pain and bulging | 1 | 0 | 1 |
| Cough | 1* | 0 | 1 |
| Urinary system infection | 1 | 0 | 1 |
| Itchy skin | 1 | 0 | 1 |
| Dizziness | 1 | 0 | 1 |
| Reduced hemoglobin | 1 | 0 | 1 |
| Increased urination at night | 0 | 1* | 1 |
| Elevated neutrophils | 0 | 1 | 1 |
| Total | 9 | 4 | 13 |
| WBC, white blood cell. * represtents severe AE. | |||