| Citation: | LIU Yong-Bei, YANG Yu-Pei, YUAN Han-Wen, LI Ming-Jiao, QIU Yi-Xing, CHOUDHARY Muhammad Iqbal, WANG Wei. A Review of Triterpenoids and Their Pharmacological Activities from Genus Kadsura[J]. Digital Chinese Medicine, 2018, 1(3): 247-258. |
Family Schisandraceae contains about 50 plant species and there are 10 species in genus Kadsura in China which mainly distributed in the southwest of China [1]. Plants from genus Kadsura have their own folk names, for instance, Kadsura coccinea (Lem.) A. C. Smith called "Heilaohu", Kadsura heteroclita (Roxb.) Craib called "Haifengteng" [2] "Xuetong" [3], and Kadsura interior A. C. Smith called "Dianjixueteng" [4]. The plants from genus Kadsura used as the folk medicines and showed good effect of activating blood and dissolving stasis, promoting Qi circulation to relieve pain, dispelling wind and eliminating dampness [5]. The numerous pharmaceutical advantages also are on-going, such as anti-tumor activity [6-8], anti-HIV [9-11], antifeedant effect [9], cytotoxic [12-13], anti-inflammatory [14-15], anti-hepatitis [16-17], nitric oxide inhibitory [18-19], anti-platelet aggregation [20-21] and neuroprotective effect [22]. The triterpenoids from genus Kadsura, mostly bearing four different skeletons: lanostane-type, kadlongilactones, cycloartane-type and schinortriterpenoids, are very important due to their bioactivities and structural diversity. This review was carried out on triterpenoids from genus Kadsura.
Triterpenoid is a kind of characteristic component in genus Kadsura. A series of highly oxygenated triterpenoids with different skeletons had been isolated from this genus. There are lots of different structure types and the following are classifications of triterpenoids from this genus.
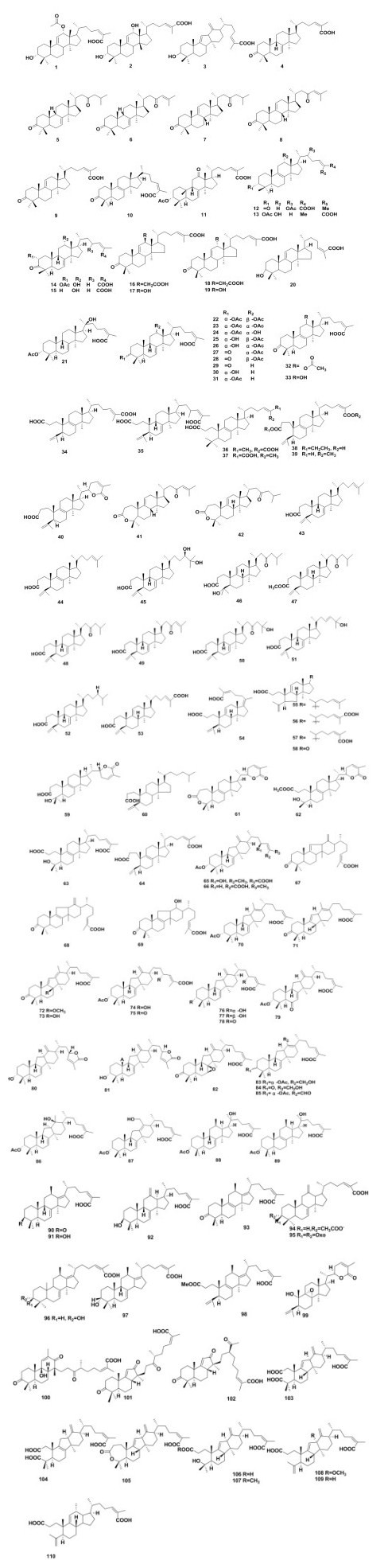
This group of compounds (1-33, Fig. 1), bearing the same skeleton, which contains the original tetracyclic core structure. Six new triterpenoids were isolated from the roots of Kadsura coccinea, named 3-hydroxy-12-acetoxycoccinic acid (1)[23]. 3-hydroxy-12-hydroxyl coccinic acid (2)[24], 3-hydroxy-neo-kadsuranic acid (3)[25], 20(R), 24(E)-3-oxo-9β-lanosta-7, 24-dien-26-oic acid (4)[26], coccinone A-D (5-8) [27], coccinic acid (9) [28], respectively. Schisanhenric acid (10) was afforded from Kadsura heteroclite [29]. Hu et al. [30] isolated one new triterpeniods from Kadsura coccinea, named: kadcoccinone F (11). Kadnanosic acid B (12) from Kadsura ananosma was separated by Yang et al. [31]. Dong et al. [32] isolated kadpolysperin L-N (13-15) from Kadsura polysperma. Li et al. [33] separated 12α-acetoxycoccinic acid (16), 12α-hydroxycoccinic acid (17), 12β-acetoxycoccinic acid (18), and 12β-hydroxycoccinic acid (19). Dai et al. [34] gained isoanwuweiziic acid (20) from Kadsura heteroclita. 11 lanostane-type triterpenoids named kadcoccinones G-Q (21-31) were isolated from Kadsura coccinea by Hu et al. [35]. (24z)-3-oxo-12α-acetoxyanosta-8, 24-dien-26-oic acid (32), (24z)-3-oxo-12α- hydroxyanosta-8, 24-dien-26-oic acid (33) were isolated from Kadsura heteroclita by Li et al. [36].
Generally, the formation of a 3, 4-lactone group or a hydroxyl group at C-2 and remain the lanostane skeleton was classified as 3, 4-seco-lanostane (34-64, Fig 1.). Four 3, 4-seco-lanostane triterpenoids, kadpolysperin H-K (34-37) were [32] isolated from Kadsura polysperma. 3-ethyl manwuweizate, 26-methyl manwuweizate from Kadsura heteroclita (38-39) were separated by Ding et al. [37]. Yang et al. [31] obtained schisanlactone F (40) from Kadsura ananosma. Coccinilactone B (41), coccinilactone A (42) and 11 new seco-triterpenoids: seco-coccinic acids A-K (43-53), kadsuracoccinic acids A-C (54-56) along with kadsuric acid (57), micranoic acid A (58), kadcoccilactones R (59) were obtained from Kadsura coccinea [27, 38-41]. Li et al. [36] isolated 3, 4-seco-(24z)- lanosta-4 (30), 24, triene-3, 26-dioic acid (60) from Kadsura heteroclita. Kadnanolactone C-D (61-62) and kadnanosic acid A (63) were separated from Kadsura ananosma [31]. Manwuweizic acid (64) was isolated from Kadsura polysperma[37].
This group of triterpenoids should come from the rearrangement of 12-OH intact lanostane (65-87, Fig. 1). Kadcoccinone A and B (65-66)[30] were separated from Kadsura coccinea. Li et al. [29] isolated neo-kadsuranic acid A-C (67-69) from Kadsura longipedunculata. Kadpolysperin A (70) was separated from Kadsura polysperma[32]. Kadcoccine acids D-E (71-73) featured as rearranged 6/6/5/6-fused triterpenoids were obtained from Kadsura coccinea. Hu et al. [42] isolated rearranged 6/6/5/6-fused triterpenoid acids A-N (74-87) from the stems of Kadsura coccinea.
It is thought that a 1, 2-methyl shift in intact lanostanes results in the occurrence of a β-oriented methyl or an exocyclic double bond at C-12 to generate 18 (13/12)-abeo-lanostane type triterpenoids (88-98, Fig. 1). Kadpolysperin C-G (88-92)[32], kadindutic acid (93)[43], ananosic acid B-C (94-95)[29], ananosic acid A, D (96-97) and kadpolysperin B (98)[44] were isolated from plants of genus Kadsura.
This group of lanostanes (99, Fig. 1), with carbon loss from the periphery of the molecules, is formed by oxidative cleavage. Yang et al. [31] isolated kadnanolactone E (99) from Kadsura ananosma.
This group of triterpenoids derived from lanostanes, which have not been classified specify (100-110, Fig. 1). Kadcoccitone C (100) along with kadcotriones A-C (101-103) possess a novel tricyclic ring system unit [45]. Liang et al. [46] isolated eight novel structure triterpene acids with 6/6/5/6-fused tetracyclic ring system unit, named: kadcoccinic acids A-C, G-I (104-109) and seco-neokadsuranic acid A (110), respectively.
In recent years, a series of kadlongilactones with novel structures have been isolated and identified (111-132, Fig. 2). Kadlongilactone C-E(111-113)and longipedlactone D, G-I (114-117) were separated from Kadsura longipedunculata[8, 47-48]. Pu et al. [10] isolated longipedlactone J (118) from Kadsura heteroclita. Gao et al [41] reported 14 new triterpenoids which were isolated from the ethyl acetate extract of the stem of Kadsura coccinea for the first time, named kadcoccilactones K-P (119-124), kadlongilactones A-B, D (125-127) and longipedlactones A-C, E-F (128-132), repectively.
This category of cycloartane-type triterpenoids featured a hydroxyl group or a ketone at C-3 (133-137, Fig. 3). Wang et al [12] isolated cycloatenone acid (133) from Kadsura heteroclita. Four intact cycloartane triterpenoids: schisandronic acid (134), heteroclic acid (135), kadsulactone (136) and 24-methylenecycloartenone (137) were obtained from the ethyl acetate extract of the stem of Kadsura coccinea [28, 41, 49].
This group of triterpenoids is abundant in genus Kadsura. The formation of a 3, 4-lactone group or a hydroxyl group at C-2 and remain the cycloartane skeleton was concluded to 3, 4-secocycloartane triterpenoids (138-176, Fig. 3). Ten new seco-cycloartanes compounds, kadcoccilactone Q (138), kadsudilacton (139), coccinetane A-H (140-147), were isolated from Kadsura coccinea [41, 49-50]. Changnanic acid (148), heteroclitalactone (A-E) (149-153), schisanlactone B (154), nigranoic acid (155), and kadsuranic acid A (156) were isolated from Kadsura heteroclita[8, 12]. Sun et al. [51] gained schisanlactone A (157) from Kadsura longipedunculata. Wang et al. [52] isolated heteroclitalactone G-L (158-163) and heteroclitalactone M (164) from Kadsura heteroclita. Gao et al. [41] seperated kadcoccilactone Q (165) from Kadsura coccinea. Chen et al. [53] isolated renchanglactone A (166) from Kadsura renchangiana. Angustific acid A-B (167-168), angustifodilactone A-B (169-170) were obtained from Kadsura angustifolia [11]. Ancilactone A-C (171-173)[54] from Kadsura lancilimba, kadsuphilactone B (174) from Kadsura philippinensis [55] andpolysperlactones A-B (175-176) from Kadsura polysperma [56] were reported in different papers.
Kadheterilactone A-B (177-178) from Kadsura heteroclita[8] and longipedlactone K-P (179-184) from Kadsura ananosma [13] were deduced as 14(13→12)-abeo-cycloartanes (Fig. 3).
The 3, 4:9, 10-disecocycloartanes compounds, kadsuphilaotone A (185) [55] and kadnanolactone A (186)[31] from Kadsura ananosma [57], and kadcoccilactone A, C (187-188) and kadcoccilactone G (189) from Kadsura coccinea were isolated and identified.
A series of highly oxygenated triterpenoids with unusual schinortriterpenoid skeletons were isolated from plants from this genus (190-203, Fig. 4). Gao et al. [57] obtained 7 new compounds from the stem of the Kadsura coccinea, kadcoccilactones B, D, E-F, H-J (190-196). Yang et al. [31] isolated kadnanolactone G-I (197-199), micrandilactone C and B (200-201), and wuweizidilactone H (202) from Kadsura ananosma. Micrandiactone H (203) was isolated from the ethyl acetate extract of the rhizome of Kadsura coccinea by Yeon et al. [58].
Other triterpenoids have not been classified clearly and most of the discovered triterpenoids were supposed to derive from cycloartanes (204-214, Fig. 5). Kadcoccinone C (204)[30] derived from lanostane triterpenoid, featured a carbon skeleton with 6/6/9-fused carbocyclic core containing a rare oxabicyclo [4.3.1] decare system, another 2 compounds kadcoccinone D-E (205-206) were provided with 6/6/5/6-fused tetracyclic ring system unit. Liang et al. [59] isolated 2 new triterpenoids kadcoccitone A-B (207-208) which featured an unprecedented carbon skeleton with a 6/6/5/5-fused tetracyclic ring system unit and a C-9side chain [45]. Zhou et al. isolated some triterpenoids from Kadsura interior and Kadsura heteroclite [60], β-amyrin (209), germanicol C (210), α-amyrin (211), ursolic acid (212), lupenol (213), lupenone (214).
Seco-coccinic acid A-C (43-45) and E (47) [38] significantly inhibited the proliferation against human leukemia HL-60 cells with IC50 value of 6.8 to 42.1 μM. Seco-coccinic acids F, G and K (48-49, 53) [39] showed cell growth inhibitory effects against human leukemia HL-60 cells with IC50 values of 16.6, 15.2 and 28.4 μM, respectively. Kadlongilactones A-B (125-126) from Kadsura coccinea [41] and Kadsura longipedunculata [61] both presented significant cytotoxicity on Leukemia K562 cells, human hepatocellular carcinoma Bel-7402 cells and human lung adenocarcinoma A549 cells, IC50 were less than 0.1, 0.1 and 1.0 μM, respectively. Kadlongilactone C, D, A and E (111, 113-114, 125) [62] showed significant cytotoxicity against 3 cancer cells (A549, HT-29, K562) with IC50 range from 0.49 to 3.60 μM. Heteroclitalactone D (152) [12] with significant cytotoxicity against HL-60 cells, and its IC50 value was 6.8 μM. Kadcoccinones A-F (11, 65-66, 204-206)[30] exhibited cytotoxicity against human cancer cell lines (HL-60, SMMC7721, A-549, MCF-7, SW-480 and Hela) by the MTT method. Structure-activity relationship of kadcoccinone A and B (65-66) indicated that the cleavage and hemiketal reactions between C-12 and C-14 might decrease the potency for cytotoxic activities.
Schisandronic acid (134) showed moderate inhibitory activity, and with the inhibition rate (56.1±8.8) %. Kadsuranic acid A (156) and nigranoic acid (155) [8] showed a strong inhibitory effect against HIV-1 protease. Kadsuphilactone B [55] presented anti-HBV activity with IC50 value at 6.0 μM. Angustific acid A-B (167-168) and angustifodilactone A-B (169-170) [11] showed anti-HIV activity determined by infected C8166 cells. The result displayed that angustific acid A presented high anti-HIV activity with IC50 6.1 μM. Kadcotriones A and C (101, 103) [45] exhibited weak anti-HIV1 activity. Kadcoccitone B (208) and 12β-hydroxycoccinic acid (19) [60] showed weak anti-HIV activity.
In studies of the cytotoxicity of Kadsura triterpenoids, kadcoccilactone N (122) and kadcoccilactone O (123) showed significant cytotoxic activity against the human K562 tumor cell line, with an IC50 value of 0.4 μM, and kadcoccilactone O exhibited potential inhibitory activity against Bel-7402, with an IC50 value of 0.8 μM [40]. Compared with lanostanes and cycloartanes, most schinortriterpenoids did not show any cytotoxicity.
Sun et al. [63] had evaluated the ability of antioxidant activity in fruits from Kadsura coccinea by the method of DPPH. The results showed that phenolic acid exhibited significant antioxidant activities and the fruit has a high nutritional value.
Genus Kadsura, economically and medicinally important plants, some of which were used as Tujia ethnomedicine for a long time. For the study of chemical constituents, only about 10 species were investigated in-depth. Triterpenoids in the plants of genus Kadsura possessed good activities of anticancer, antiviral and antioxidant activities. We tried to summarize triterpenoids from this genus and 11 species have been mentioned in structure part, leading to the isolation and identification of more than 200 triterpenoids, some of which exhibited unprecedented structural skeletons and exciting bioactivities, which has brought great interests and challenges for phytochemists and pharmacologists. Therefore, we need a more comprehensive study of genus Kadsura, in order to clarify its mechanism in vitro and provide a scientific basis on the further development and use of this genus plants. And also, it is necessary to further carry out the chemical and pharmacological study on the plants from genus Kadsura.
We thank for the funding support from Hunan Province Universities 2011 Collaborative Innovation Center of Protection and Utilization of Huxiang Chinese Medicine Resources, Hunan Provincial Key Laboratory of Diagnostics in Chinese Medicine, and National Natural Science Foundation of China (No. 81673579).
The authors declare no conflict of interest.
| [1] |
Committee of Chinese Academy of Sciences Flora of China. Flora of China (Volume 30(1)). Beijing: Science Press, 1996.
|
| [2] |
WANG C C, MAO Z H. Study on the varieties and quality of P. puberulum(Benth.)Maxim. Journal of Clinical Medical Literature (Electronic Edition), 2015, 2(8):1578-1579.
|
| [3] |
SU W, ZHAO J, YANG M, et al. A coumarin lignanoid from the stems of Kadsura heteroclita. Bioorganic & Medicinal Chemistry Letters, 2015, 25(7):1506-1508. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0234659893
|
| [4] |
GAO S M, GUO H J, QI Y D, et al. Study on material basis of stems of Kadsura interior A. C. Smith. Journal of Chinese Medicinal Materials, 2015, 38(12):2644-2650.
|
| [5] |
LIU J, QI Y, LAI H, et al. Kadsura species, a good source with considerable characteristic chemical constituents and potential bioactivities. Phytomedicine International Journal of Phytotherapy & Phytopharmacology, 2014, 21(8/9):1092-1097.
|
| [6] |
GAO X M, PU J X, HUANG S X, et al. Kadcoccilactones A-J, Triterpenoids from Kadsura coccinea. Journal of Natural Products, 2008, 71(7):1182-1188. doi: 10.1021/np800078x
|
| [7] |
DAO F X, SHUN X Z, MUSTSUO K, et al. Interiotherins C and D, Two New Lignans from Kadsura interior and Antitumor-Promoting Effects of Related Neolignans on Epstein-Barr Virus Activation. Journal of Natural Products, 2002, 65(9):1242-1245. doi: 10.1021/np0105127
|
| [8] |
XU L J, PENG Z G, ChEN H S, et al. Bioactive Triterpenoids from Kadsura heteroclita. Chemistry & Biodiversity, 2010, 7(9):2289–2295. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0220778603/
|
| [9] |
LUO X, SHI Y M, LUO R H, et al. Schilancitrilactones A–C: Three Unique Nortriterpenoids from Schisandra lancifolia. Organic Letters, 2012, 14(5):1286-1289. doi: 10.1021/ol300099e
|
| [10] |
PU J X, YANG L M, XIAO W L, et al. Compounds from Kadsura heteroclita and related anti-HIV activity. Phytochemistry, 2008, 69(5):1266-1272. doi: 10.1016/j.phytochem.2007.11.019
|
| [11] |
SUN R, SONG H C, WANG C R, et al. Compounds from Kadsura angustifolia with anti-HIV activity. Bioorganic & Medicinal Chemistry Letters, 2011, 21(3):961-965. http://www.sciencedirect.com/science/article/pii/S0960894X10018196
|
| [12] |
WANG W, LIU J, HAN J, et al. New triterpenoids from Kadsura heteroclita and their cytotoxic activity. Planta Medica, 2006, 72(5):450-457. doi: 10.1055/s-2005-916263
|
| [13] |
YANG J H, PU J X, WEN J, et al. Cytotoxic triterpene dilactones from the stems of Kadsura ananosma. Journal of Natural Products, 2010, 73(1):12-16. doi: 10.1021/np900506g
|
| [14] |
LIN L C, SHEN C C, SHEN Y C, et al. Anti-inflammatory Neolignans from Piper Kadsura. Journal of Natural Products, 2006, 69(5):842-844. doi: 10.1021/np0505521
|
| [15] |
KIM, K H, CHOI, J W, HA S K, et al. Neolignans from Piper Kadsura and their anti-neuroinflammatory activity. Bioorganic & Medicinal Chemistry Letters, 2010, 20(1):409-412. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0215639425/
|
| [16] |
KUO Y H, WU M D, HUANG R L, et al. Antihepatitis activity (anti-HBsAg and anti-HBeAg) of C19 homolignans and six novel C18 dibenzocyclooctadiene lignans from Kadsura japonica. Planta Medica, 2005, 71(7):646-653. doi: 10.1055/s-2005-871271
|
| [17] |
KUO YH, WU MD, HUNG CC, et al. Syntheses of C (18) dibenzocyclooctadiene lignan derivatives as anti-HBsAg and anti-HBeAg agents. Bioorganic & Medicinal Chemistry, 2005, 13(5):1555-1561. http://europepmc.org/abstract/MED/15698772
|
| [18] |
MULYANINGSIL S, YOUNNS M, El-Readi M Z, et al. Biological activity of the essential oil of Kadsura longipedunculata, (Schisandraceae) and its major components. Journal of Pharmacy and Pharmacology, 2010, 62(8):1037-1044. doi: 10.1111/j.2042-7158.2010.01119.x
|
| [19] |
Awale S, Tezuka Y, Banskota A H, et al. Nitric oxide inhibitory isopimarane-type diterpenes from Orthosiphon stamineus of Indonesia. Journal of Natural Products, 2003, 66(2):255-258. doi: 10.1021/np020455x
|
| [20] |
Shi Y M, Wang X B, Li X N, et al. Lancolides, antiplatelet aggregation nortriterpenoids with tricyclo[6.3.0.0(2, 11)]undecane-bridged system from Schisandra lancifolia. Organic Letters, 2013, 15(19):5068-5071. doi: 10.1021/ol402414z
|
| [21] |
Yan L, Chen D F, Xie P S, et al. Analysis of Schisandra chinensis and Schisandra sphenanthera. Journal of Chromatography A, 2009, 1216(11):1980-1990. doi: 10.1016/j.chroma.2008.09.070
|
| [22] |
Dong K, Pu J X, Zhang H Y, et al. Dibenzocyclooctadiene lignans from Kadsura polysperma and their antineurodegenerative activities. Journal of Natural Products, 2012, 75(2):249-256. doi: 10.1021/np200937h
|
| [23] |
Song Y, Zhao Q J, Jin Y S, et al. A new triterpenoid from Kadsura coccinea. Chinese Chemical Letters, 2010, 21(11):1352-1354. doi: 10.1016/j.cclet.2010.06.027
|
| [24] |
Liu J S, Li L. Schisantherins L-O and acetylschisantherin L from Kadsura coccinea. Phytochemistry, 1993, 32(32):1293-1296. http://www.sciencedirect.com/science/article/pii/S0031942200951082
|
| [25] |
Yan S, Zhao Q J, Jin Y S, et al. Two new triterpenoid acids from Kadsura coccinea. Archives of Pharmacal Research, 2010, 33(12):1933-1936. doi: 10.1007/s12272-010-1207-0
|
| [26] |
Ban N K, Thanh B V, Kiem P V, et al. Dibenzocyclooctadiene lignans and lanostane derivatives from the roots of Kadsura coccinea and their protective effects on primary rat hepatocyte injury induced by t-butyl hydroperoxide. Planta Medica, 2009, 75(11):1253-1257. doi: 10.1055/s-0029-1185537
|
| [27] |
Wang N, Li Z L, Li D Y, et al. Five New Triterpenoids from the Roots of Kadsura coccinea. Helvetica Chimica Acta, 2009, 92(7):1413-1418. doi: 10.1002/hlca.v92:7
|
| [28] |
Li L N, Xue H. Triterpenoids from Roots and Stems of Kadsura coccinea. Planta Medica, 1987, 52(6):492-493. http://europepmc.org/abstract/MED/17345419
|
| [29] |
Hu Z X, Li X N, Shi Y M, et al. Lanostane-type triterpenoids from Kadsura coccinea. Tetrahedron, 2017, 73(20):2931-2937. doi: 10.1016/j.tet.2017.03.087
|
| [30] |
Li L N, Xue H, Kangouri K, et al. Triterpenoid Acids from Kadsura longipedunculata. Neokadsuranic Acids B and C: Two Novel Triterpenoids with 14 (13/12) abeo-Lanostane Skeletons. Planta Medica, 1989, 55(3):294-296. doi: 10.1055/s-2006-962010
|
| [31] |
Hu Z X, Shi Y M, Wang W G, et al. Kadcoccinones A–F, new biogenetically related lanostane-type triterpenoids with diverse skeletons from Kadsura coccinea. Organic Letters, 2015, 17(18):4616-4619. doi: 10.1021/acs.orglett.5b02360
|
| [32] |
Yang J H, Jin W, Xue D, et al. Triterpenoids from the stems of Kadsura ananosma. Tetrahedron, 2010, 66(46):8880-8887. doi: 10.1016/j.tet.2010.09.059
|
| [33] |
Dong K, Pu J X, Du X, et al. Kadpolysperins A–N, lanostane triterpene acids possessing rich structure types from Kadsura polysperma. Tetrahedron, 2012, 68(24):4820-4829. doi: 10.1016/j.tet.2012.03.116
|
| [34] |
Li LN, Xue H, Kunio K, et al. Isolation and Structure Elucidation of 12β-Acetoxycoccinic Acid, 12β-Hydroxycoccinic Acid, 12α-Acetoxycoccinic Acid, and 12α-Hydroxycoccinic Acid. Planta Medica, 1989, 55(6):548-550. doi: 10.1055/s-2006-962091
|
| [35] |
Dai P, Han G, Arison B H. A New Triterpenoid form Kadsura heteroclita (Roxb)Craib. Chemical Research in Chinese Universities, 1990, 11(4):423-424. http://en.cnki.com.cn/article_en/cjfdtotal-gdxh199004022.htm
|
| [36] |
Li L N, Xue H, Da L G, et al. Isolation and Structure Elucidation of Seco-neokadsuranic Acid A and 3, 4-Seco-(24z)-lanosta-4(30), 8, 24-triene-3, 26-dioic Acid. Planta Medica, 1989, 55(3):300-302. doi: 10.1055/s-2006-962012
|
| [37] |
Ding X, Yan P, Li J, et al. Two New 3, 4-Seco-Lanostane Triterpenoids from Stems of Kadsura heteroclita. Chinese Herbal Medicines, 2015, 7(3):283-286. doi: 10.1016/S1674-6384(15)60052-1
|
| [38] |
Wang N, Li Z, Song D, et al. Lanostane-type triterpenoids from the roots of Kadsura coccinea. Journal of Natural Products, 2008, 71(6):990-994. doi: 10.1021/np7007522
|
| [39] |
Wang N, Li Z L, Song D D, et al. Five new 3, 4-Seco-lanostane-type triterpenoids with antiproliferative activity in human leukemia cells isolated from the roots of Kadsura coccinea. Planta Medica, 2012, 78(78):1661-1666. http://europepmc.org/abstract/MED/22948612
|
| [40] |
Li H, Wang L, Miyata S, et al. Kadsuracoccinic acids A-C, ring-A Seco-lanostane triterpenes from Kadsura coccinea and their effects on embryonic cell division of Xenopus laevis. Journal of Natural Products, 2008, 71(4):739-741. doi: 10.1021/np700739t
|
| [41] |
Gao X M, Pu J X, Xiao W L, et al. Kadcoccilactones K–R, triterpenoids from Kadsura coccinea. Cheminform, 2009, 64(18):11673-11679. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ028942705/
|
| [42] |
Hu Z X, Hu K, Shi Y M, et al. Rearranged 6/6/5/6-Fused Triterpenoid Acids from the stems of Kadsura coccinea. Journal of Natural Products, 2016, 79(10): 2590-2598. doi: 10.1021/acs.jnatprod.6b00508
|
| [43] |
Ma W, Ma X, Lu Y, et al. Lignans and triterpenoids from the stems of Kadsura induta. Helvetica Chimica Acta, 2009, 92(4):709-715. doi: 10.1002/hlca.v92:4
|
| [44] |
Chen Y G, Xie Y Y, Cheng K F, et al. Compounds from Kadsura ananosma. Phytochemistry, 2001, 58(8):1277-1280. doi: 10.1016/S0031-9422(01)00319-3
|
| [45] |
Liang C Q, Shi Y M, Li X Y, et al. Kadcotriones A-C: tricyclic triterpenoids from Kadsura coccinea. Journal of Natural Products, 2013, 76(12):2350-2354. doi: 10.1021/np400546z
|
| [46] |
Liang C Q, Shi Y M, Wang W G, et al. Kadcoccinic Acids A-J, Triterpene acids from Kadsura coccinea. Journal of Natural Products, 2015, 78(8):2067-2073. doi: 10.1021/acs.jnatprod.5b00392
|
| [47] |
Pu J X, Xiao W L, Lu Y, et al. Kadlongilactones A and B, two novel triterpene dilactones from Kadsura longipedunculata possessing a unique skeleton. Organic Letters, 2005, 7(22):5079-5082. doi: 10.1021/ol052161j
|
| [48] |
Pu J X, Li R T, Xiao W L, et al. Longipedlactones A–I, nine novel triterpene dilactones possessing a unique skeleton from Kadsura longipedunculata. Cheminform, 2006, 62(41):6073-6081. http://www.sciencedirect.com/science/article/pii/S0040402006005394
|
| [49] |
Tan R, Xue H, Li L N. Kadsulactone and kadsudilactone, two new triterpenoid lactones from Kadsura species. Planta Medica, 1991, 57(1):87-88. doi: 10.1055/s-2006-960031
|
| [50] |
Sy L K, Brown G D. Novel Seco-cycloartanes from Kadsura coccinea and the assisted autoxidation of a tri-substituted alkene. Tetrahedron, 1999, 55(1):119-132. doi: 10.1016/S0040-4020(98)01021-7
|
| [51] |
Sun Q Z, Chen D F, Ding P L, et al. Three New Lignans, Longipedunins A–C, from Kadsura longipedunculata and Their Inhibitory Activity Against HIV-1 Protease. Cheminform. 2006, 54(1):129-132. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ027547786/
|
| [52] |
Wang W, Xu Z, Yang M, et al. Structural determination of seven new triterpenoids from Kadsura heteroclita by NMR techniques. Magnetic Resonance in Chemistry, 2007, 45(6):522-526. doi: 10.1002/(ISSN)1097-458X
|
| [53] |
Chen M, Chen D F. Renchanglactone A, a new triterpenoid lactone from Kadsura renchangiana. Natural Product Research, 2008, 22(3):203-207. doi: 10.1080/14786410600905964
|
| [54] |
Chen D F, Shun X Z, Wang H K, et al. Novel Anti-HIV Lancilactone C and Related Triterpenes from Kadsura lancilimba. Journal of Natural Products, 1999, 62(1):94-97. doi: 10.1021/np980291d
|
| [55] |
Shen Y C, Lin Y C, M Y C, et al. Kadsuphilactones A and B, Two New Triterpene Dilactones from Kadsura philippinensis. Organic Letters, 2005, 7(15):3307-3310. doi: 10.1021/ol051155k
|
| [56] |
Jia Z, Lu Y, Liao Z, et al. Two New Triterpene Lactones from the Stems of Kadsura polysperma. Helvetica Chimica Acta, 2007, 90(6):1236-1243. doi: 10.1002/(ISSN)1522-2675
|
| [57] |
Gao X M, Pu J X, Huang S X, et al. Kadcoccilactones A-J, Triterpenoids from Kadsura coccinea. Journal of Natural Products, 2008, 71(7):1182-1188. doi: 10.1021/np800078x
|
| [58] |
Yeon J H, Cheng L, He Q Q, et al. A lignin glycoside and a nortriterpenoid from Kadsura coccinea. Chinese Journal of Natural Medicines, 2014, 12(10):782-785. doi: 10.1016/S1875-5364(14)60119-9
|
| [59] |
Liang C Q, Shi Y M, Luo R H, et al. Kadcoccitones A and B, two new 6/6/5/5-fused tetracyclic triterpenoids from Kadsura coccinea. Organic Letters, 2012, 14(24):6362-6365. doi: 10.1021/ol303168y
|
| [60] |
Zhou S Y, Li R T, Li H M. Study on Chemical Constituents of Kadsura Interior. Journal of Kunming University of Science and Technology, 2008, 33(5):81-85. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kmlgdxxb200805020
|
| [61] |
Pu J X, Huang S X, Ren J, et al. Isolation and structure elucidation of kadlongilactones C-F from Kadsura longipedunculata by NMR spectroscopy and DFT computational methods. Journal of Natural Products, 2007, 70(11):1706-1711. doi: 10.1021/np070247a
|
| [62] |
Ye G C, Li N H, Xin R L, et al. Ananosic Acids B and C, Two New 18(13→12)-abeo-Lanostane Triterpenoids from Kadsura ananosma. Journal of Natural Products, 2004, 67(5):875-877. doi: 10.1021/np0340302
|
| [63] |
Sun J, Yao J, Huang S, et al. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsura coccinea (Lem.) A.C. Smith. Food Chemistry, 2009, 117(2):276-281. doi: 10.1016/j.foodchem.2009.04.001
|
| 1. | Chang, K.-H., Chen, C.-M. The Role of NRF2 in Trinucleotide Repeat Expansion Disorders. Antioxidants, 2024, 13(6): 649. DOI:10.3390/antiox13060649 | |
| 2. | Zeng, N., Su, W., Zhang, Q.-D. et al. Chemical constituents from roots of Kadsura heteroclita | [异型南五味子根的化学成分研究]. Zhongguo Zhongyao Zazhi, 2024, 49(6): 1549-1557. DOI:10.19540/j.cnki.cjcmm.20231212.203 | |
| 3. | Noguchi, K., Saito, S., Yamakoshi, H. et al. Stereoselective Synthesis of the DE Ring Portion of Kadcoccilactone A by a Radical Addition/Cyclization Approach. European Journal of Organic Chemistry, 2022, 2022(45): e202201201. DOI:10.1002/ejoc.202201201 | |
| 4. | Wang, M., Jiang, S., Hussain, N. et al. Anti-RAFLS Triterpenoids and Hepatoprotective Lignans From the Leaves of Tujia Ethnomedicine Kadsura heteroclita (Xuetong). Frontiers in Chemistry, 2022. DOI:10.3389/fchem.2022.878811 | |
| 5. | Yang, Y., Zhang, X., Liu, L. et al. Phytochemical and chemotaxonomic studies on the stems and leaves of Schisandra chinensis (Turcz.) Baill. Biochemical Systematics and Ecology, 2021. DOI:10.1016/j.bse.2021.104328 | |
| 6. | Xu, J., Wei, X.-P., Liu, J.-S. et al. Genome sizes of four important medicinal species in Kadsura by flow cytometry. Chinese Herbal Medicines, 2021, 13(3): 416-420. DOI:10.1016/j.chmed.2021.05.002 | |
| 7. | Ghiulai, R., Roşca, O.J., Antal, D.S. et al. Tetracyclic and pentacyclic triterpenes with high therapeutic efficiency in wound healing approaches. Molecules, 2020, 25(23): 5557. DOI:10.3390/molecules25235557 | |
| 8. | Yang, Y., Liu, Y., Daniyal, M. et al. New Lignans from roots of Kadsura coccinea. Fitoterapia, 2019. DOI:10.1016/j.fitote.2019.104368 | |
| 9. | Huang, S.Z., Duan, L.P., Wang, H. et al. Two New AChE Inhibitors Isolated from Li Folk Herb Heilaohu “Kadsura coccinea” Stems. Molecules, 2019, 24(19): 3628. DOI:10.3390/molecules24193628 | |
| 10. | Zhang, M., Zhou, J.-Y., Wei, W. et al. Research progress on chemical constituents and pharmacological effects of Yao medicine Kadsura heteroclita | [瑶药大红钻化学成分及药理作用研究进展]. Chinese Traditional and Herbal Drugs, 2019, 50(14): 3493-3502. DOI:10.7501/j.issn.0253-2670.2019.14.033 |