| Citation: | SHEN Bing-Bing, YANG Yu-Pei, Yasamin Sumera, LIANG Na, SU Wei, CHEN Sheng-Huang, WANG Xiao-Juan, WANG Wei. Analysis of the Phytochemistry and Bioactivity of the Genus Polygonum of Polygonaceae[J]. Digital Chinese Medicine, 2018, 1(1): 19-36. |
The genus Polygonum (Polygonaceae) includes approximately 300 species that are widely distributed around the world, mostly in north temperate regions. Among 113 Polygonum species in China [1], Polygonum cuspidatum Sieb et Zucc., P. aviculare L., P. bistorta L., and P. flaccidum Meissn. have been used as traditional Chinese medicines, as described in the Chinese Pharmacopoeia. Additionally, P. orientale L., P. tinctorium, P. persicaria L., and P. runcinatum have been shown to have good effects as traditional Chinese medicines.
Studies of the chemical constituents and pharmacological activities of Polygonum plants began at the end of the last century. In recent years, with the rapid development of chromatographic separation technologies and experimental methods, phytochemical investigations have indicated the presence of flavonoids, quinones, phenylpropanoids, and terpenoids in the genus Polygonum. In addition, crude extracts and pure compounds from this genus have been reported to exhibit a wide array of bioactivities, including anticancer, antitumor, anti-oxidative, anti-inflammatory, analgesic, antimicrobial, and insecticidal activities.
In this review, we provide an overview of the literature describing different classes of compounds isolated from the genus Polygonum in the last 15 years, with a focus on their reported biological activities.
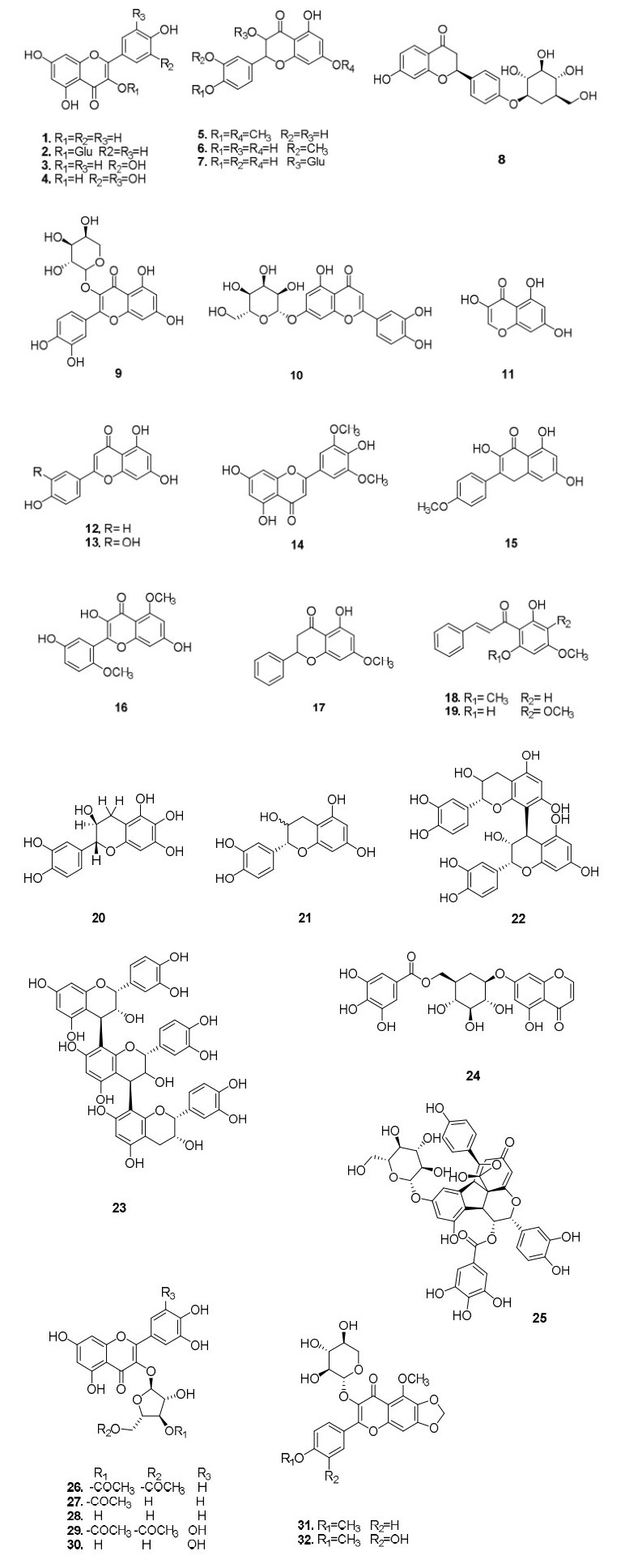
Flavonoids are the main secondary metabolites in Polygonum (Polygonaceae) species, structures of which were shown in Figure 1. Kaempferol (1), baicalin (2), quercetin (3), myricetin (4), 7, 4'-dimethyl quercetin (5), 3-methyl quercetin (6), and isoquercetin (7) have been isolated from different species of the genus Polygonum, including P. cuspidatum Sieb et Zucc., P. runcinatum, P. perfoliatum L., P. bistorta L., and P. aviculare L. Li [1] isolated kaempferol, quercetin, myricetin3-0-(3" -0- galloy1- rhamnopyranoside, and juglanin from P. aviculare L [2]. Additionally, myricetin3-O-(3" -O-galloy1- rhamnopyranoside was isolated from Polygonum species, and Yunuskhodzhaeva [3] isolated liquiritin (8), avicularin (9), and cinaroside (10) from P. aviculare L. Lin [4] isolated quercetin-3-O-β-d- galactoside, 8-methoxyl quercetin, 3, 5, 7-trihydroxy chromone (11), apigenin (12), and luteolin (13) from P. jucundum. Peng [5] isolated tricin (14) and 7, 4-dimethoxy kaempferol (15) from P. sieboldii Meisn. Partovi [6] isolated a flavonol with coagulant properties, i.e., 3, 7, 3-trihydroxy-5, 6- dimethoxyflavone (16), from P. bistorta. Kurkina [7] isolated 5-hydroxy-7- methoxyflavanone (17), 6′-hydroxy-2′, 4′-dimethoxy-chalcone (18), and 2′, 6′-dihydroxy-4′, 5′-dimethoxychalcone (19) from P. persicaria. Tantry [8] isolated a new flavone identified as (2R, 3S)-3', 4', 5, 6, 7-pentahydroxyflavan-3-ol (20) from P. amplexicaule. Wang [9] isolated epicatechin (21), epicatechin dimers (22), and epicatechin trimers (23) from P. paleaceum, and Nikolaeva [10] isolated rutin, hyperoside, luteolin-7-glycoside, isoquercetin, avicularin, cosmosiin, and kaempferol glycoside from aerial parts of four Polygonum species (P. divaricatum, P. angustifolium, P. amphibium, and P. aviculare) by high-performance liquid chromatography (HPLC). Li [11] isolated a new chromone glycoside 7-O-(6′-galloyl)-β-d-glucopyranosyl-5-hydroxychromone (24) from P. capitatum, and Li [12] isolated polyflavanostilbene A (25), a new flavanol-fused stilbene glycoside, from P. cuspidatum.
Moradi-Afrapoli [13] isolated five phenolic compounds, including quercetin 3-O-α-l- (3" , 5" -diacetyl-arabinofuranoside) (26), quercetin 3-O-α-l-(3" -acetyl-arabinofuranoside) (27), avicularin (28), myricetin 3-O-α-l-(3" , 5" -diacetyl- arabinofuranoside) (29), and myricetin 3-O-α-l- arabinofuranoside (30) from aerial parts of P. hyrcanicum. Zheng [14] isolated two new flavone glycosides, i.e., viviparum A (31), and viviparum B (32), from P. viviparum L.
Quinones are characteristic components of Polygonum species, such as P. cuspidatum Sieb. et Zucc., P. bistorta L., P. perfoliatum, and P. hydropiper. Some Polygonum species contain chrysophanol (33), emodin (34), rhein (35), emodin-6-methyl-ether (36), aloe-emodin (37), and 6-hydroxy aloemodin (38) as major constituents as well as some other anthraquinones, anthrone, and naphthoquinone. Liu [15] isolated four anthraquinones, including emodin (34), rhein (35), physcion (39), and chrysophanone (40), as well as 2-methoxystypandrone (41), as major active components of P. cuspidatum. Shu [16] isolated three anthraquinones from Rumex japonicus by high-speed counter-current chromatography, and Al-Hazimi [17] isolated four naphthoquinones, including sitosterol (42), oleanolic acid (43), 5, 6, 7, 40-tetramethoxyflavanone (44), and 6-methoxy plumbagin (45). Notably, 6-methoxyplumbagin (42) was also isolated from the acetone extracts of P. aviculare. Zhang [18] isolated two new anthraquinone malonyl glucosides (46 and 47) from P. cuspidatum.
Few reports have described phenylpropanoid compounds of the genus Polygonum (Polygonaceae). Sun [19] isolated five compounds, including 6-acetyl-3, 6-diferuloylsucrose (48), 2, 4, 6-triacetyl-3-6-diferuloylsucrose (49), 1, 2, 4, 6-tetraacetyl-3, 6-diferuloylsucrose (50), 1, 2, 6-triacetyl-3, 6-diferuloyl-sucrose (51), and 2, 6-diacetyl-3, 6-diferuloylsucrose (52), from P. perfoliatum L., and Wang [20] isolated a new lignan, 8-oxo-pinoresinol (53), from the methanol extract of the tubers of P. perfoliatum L. Xiao [21] isolated two sulfate lignans, sodium(-)-lyoniresinol-2α-sulfate (54) and sodium(+)-isolaricireinol-2α-sulfate (55), from aqueous extracts of the roots of P. cuspidatum. Structures (33-55) were shown in Figure 2.
Cheng [22] and Li [23] isolated five triterpenoids and a diterpene, i.e., (24S)-24-ethylcholesta-3β, 5α, 6α-triol (56), cucurbitacin Ⅱa (57), cucurbitacin U (58), asteryunnanoside F (59), saikosaponin M (60), and 13-hydroxy helianthemum 8-(17), 14-diene-19- aldehyde (61), from ethyl acetate extracts of P. perfoliatum. Sun [24] isolated five triterpenoids, i.e., 3β-acetoxy-dammara-20, 24-diene (62), arborinone (63), adianenone (64), arborinol (65), and isoarborinol (66), from the dried roots of P. bistorta L. Compounds 62–66 were isolated from the Polygonum genus for the first time. Mazid [25] identified sitosterone (67) from the petroleum ether fraction and viscozulenic acid (68) from the chloroform fraction of the methanol extracts of P. barbatum L. Bidyut [26] isolated three new sesquiterpenes, i.e., iscozulenic acid methylester (69), viscoazucinic acid (70), and polygosumic acid (71), from the chloroform extracts of aerial parts of P. viscosum by reversed-phase preparative HPLC. Additionally, Yang [27] isolated a new terpenoid saponin, 8-O-β-d-glucopyranosyl-3β, 7β-dihydroxy-lup-20(29)-en-28-oate (72), from the fruits of P. orientale. Bidyut [28] isolated a sesquiterpene acid, viscosumic acid (73) from P. viscosum. Karuppiah [29] isolated two cycloartane-type triterpenoids, i.e., 24(E)-ethylidenecycloartanone (74) and 24(E)-ethylidenecycloartan-3α-ol (75), from the rhizomes of P. bistorta. Terpenoids' structures were shown in Figure 3.
Some other compounds from different classes have been isolated from the genus Polygonum (Polygonaceae), as shown in figure 4. Among these compounds, stilbene glycosides are major components found in P. multiflorum. Xiao [30] isolated 10 stilbene glycosides (76–85), and Li [31] isolated five new compounds (86–90). Xiang [32] isolated 12 phenolic compounds from P. amplexicaule var. Sinense, and Takasaki [33, 34] isolated four new phenyl propanoid esters of sucrose, namely, lapathoside A (91), lapathoside B (92), lapathoside C (93), and lapathoside D (94), from aerial parts of P. lapathifolium together with the known esters vanicoside B (95) and hydropiperoside (96). Liu [35] isolated a new tannin-related compound identified as 3-methyl-gallic acid 4-O-β-d-(6'-O-3" -methyl- galloyl)-glucopyranoside (97) from the rhizome of P. bistorta L. Additionally, Li [36] isolated three alkaloids, including N-cis-feruloyltyramine (98), N-trans- feruloyltyramine (99), and paprazine (100), from flowers of P. oriental. Smolarz [37] isolated a new carboxystilbene from P. persicaria 2-carboxy-3, 5-methoxy-E-stilbene (101), and Zhou [38] isolated a new ellagic acid derivative, runcinatside (102), with four ellagic acids (103–106) from the roots of P. runcinatum. Yang [39] isolated the new compound neopaleaceolactoside (107), along with nine known compounds, from the EtOAc and n-BuOH extracts of the rhizomes of P. paleaceum. Madhukar [40] identified the new natural product (2)-2-methoxy-2-butenolide-3-cinnamate (108) from the methanol extracts of the aerial parts of P. glabrum. Wang [41] isolated 3, 3, 4′-trimethylellagic acid (109), 3, 3-di-O-methyl ellagic acid (110), 3, 3, 4-tri-O-methylellagic acid-4′-d-glucopyranoside (111), and 3, 3′-di-O-methylellagic acid-4′-O-β-d- glucopyranoside (112) from theethanol extracts of P. runcinatum. Hu [42] isolated rosemary acid (113), caffeic acid (114), and coumaric acid (115) from P. aviculane, and Zhang [43] isolated hirsutine (116) from the genus Polygonum for the first time. Zhang [44] isolated tadeonal (117) and isotadeonal (118) from P. hydropiper Linn., and Shen [45] isolated two new apianen lactones, i.e., guanyeliaoine Ⅰ (119) and guanyeliaoine Ⅱ (120), along with seven known compounds, from P. perfoliatum L. Moreover, Liu [46] isolated the new phenylpropanoid esters vanicoside A′ (121), hydropiperoside-B (122), and hydropiperoside A (123) from P. pubescens Blume.
| Plant | Compounds | Reference |
| Polygonum aviculare L. (Bian Xu, 扁蓄) |
Kaempferol | 2 |
| Myricetin | 2 | |
| Myricetin3-O-(3" -O-galloy1)-rhamnopyranoside | 2 | |
| Liquiritin | 3 | |
| Avicularin | 3 | |
| Cinaroside | 3 | |
| 6-Methoxyplumbagin | 17 | |
| Polygonum amplexicaule (Bao Jing Liao, 抱茎蓼) |
(2R, 3S)-3′, 4′, 5, 6, 7-pentahydroxyflavan-3-ol | 8 |
| Polygonum angustifolium (Xi Ye Liao, 细叶蓼) |
Avicularin | 10 |
| Polygonum amphibium (Liang Xi Liao, 两栖蓼) |
Hyperoside | 10 |
| Polygonum aviculane (Bian Xu Liao, 扁蓄蓼) |
Rosemary acid | 42 |
| Caffeic acid | 42 | |
| Coumaric acid | 42 | |
| Polygonum bistorta L. (Quan Liao, 拳蓼) |
3, 7, 3-Trihydroxy-5, 6-dimethoxyflavone | 6 |
| 3β-Acetoxy-dammara-20, 24-diene | 24 | |
| Arborinone | 24 | |
| Adianenone | 24 | |
| Arborinol | 24 | |
| 24(E)-ethylidenecycloartanone | 29 | |
| 24(E)-ethylidenecycloartan-3α-ol | 29 | |
| 3-Methyl-gallic-acid, 4-O-β-d-(6′-O-3" -methyl-galloyl)-glucopyranoside | 35 | |
| 3-Methyl-gallic-acid, 4-O-β-d-(6′-O-3" -methyl-galloyl)-glucopyranoside | 35 | |
| Polygonum barbatum L. (Mao Liao, 毛蓼) |
Sitosterone | 25 |
| Viscozulenic acid | 25 | |
| Polygonum capitatum (Tou Hua Liao, 头花蓼) |
7-O-(6′-galloyl)-β-d-glucopyranosyl-5-hydroxychromone | 11 |
| Hirsutine | 43 | |
| Polygonum cuspidatum (Hu Zhang, 虎杖) |
Polyganins A | 18 |
| Polyganins B | 18 | |
| Polyflavanostilbene A | 12 | |
| Sodium(-)-lyoniresinol-2α-sulfate | 21 | |
| Sodium(+)-isolaricireinol-2α-sulfate | 21 | |
| Polygonum divaricatum (Cha Fen Liao, 叉分蓼) |
Isoquercetin | 10 |
| Polygonum multiflorum (He Shou Wu, 何首乌) |
Chrysophanol | 15 |
| Physcion | 15 | |
| 2-Methoxystypandrone | 15 | |
| (E)-2, 3, 5, 4′-tetrahydroxystilbene-2-O-(4" -O-a-d-glucopyranosyl)-β-d-glucopyranoside | 31 | |
| (E)-2, 3, 5, 4′-tetrahydroxystilbene-2-O-(6" -O-β-d-glucopyranosyl)-β-d-glucopyranoside | 31 | |
| (E)-2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-d-glucopyranosyl-4′-O- a-d-glucopyranoside | 31 | |
| (E)-2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-d-glucopyranosyl-5-O- a-d-glucopyranoside | 31 | |
| (E)-2, 3, 5, 4′-tetrahydroxystilbene-2-O-(2" -O-β-d-fructofuranosyl)-β-d-glucopyranoside | 31 | |
| Polygonum glabrum. (Guang Liao, 光蓼) |
(2)-2-methoxy-2-butenolide-3-cinnamate | 40 |
| Polygonum hydropiper (Shui Liao, 水蓼) |
Tadeonal | 44 |
| Isotadeonal | 44 | |
| Polygonum hyrcanicum (Hua Nan Liao, 华南蓼) |
Quercetin 3-O-α-l-(3" , 5" -diacetyl arabinofuranoside) | 13 |
| Quercetin 3-O-α-l-(3" -acetyl-arabinofuranoside) | 13 | |
| Quercetin 3-O-α-l-arabinofuranoside | 13 | |
| Myricetin 3-O-α-l-(3" , 5" -diacetyl-arabinofuranoside) | 13 | |
| Myricetin 3-O-α-l-arabinofuranoside | 13 | |
| Polygonum jucundum (Yu Yue Liao, 愉悦蓼) |
3, 5, 7-three hydroxy chromone | 4 |
| Apigenin | 4 | |
| Polygonum lapathifolium (Suan MoYe Liao, 酸模叶蓼) |
Lapathosides A-D | 33 |
| Vanicoside B | 34 | |
| Hydropiperoside | 34 | |
| Polygonum orientale (Hong Liao, 红蓼) |
8-O-β-d-glucopyranosyl-3β, 7β-dihydroxy-lup-20(29)-en-28-oate | 27 |
| Polygonum oriental (Hong Cao, 荭草) |
N-cis-feruloyltyramine | 36 |
| N-trans-feruloyltyramine | 36 | |
| Paprazine | 36 | |
| Polygonum persicaria (Chun Liao, 春蓼) |
5-Hydroxy-7-methoxyflavanone | 7 |
| 6′-Hydroxy-2′, 4′-dimethoxychalcone | 7 | |
| 2′, 6′-Dihydroxy-4′, 5′-dimethoxychalcone | 7 | |
| 2-Carboxy-3, 5-methoxy-E-stilbene | 37 | |
| Polygonum paleaceum (Cao Xue Jie, 草血竭) |
Epicatechin | 9 |
| Procyanidin B-C | 9 | |
| Neopaleaceolactoside | 39 | |
| Polygonum perfoliatum L. (Gang Ban Gui, 杠板归) |
6-Acety1-3, 6-diferuloylsucrose | 19 |
| 2, 4, 6-Triacetyl-3-6-diferuloylsucrose | 19 | |
| 1, 2, 4, 6-Tetraacetyl-3, 6-diferuloylsucrose | 19 | |
| 1, 2, 6-Triacetyl-3, 6-diferuloylsucrose | 19 | |
| 2, 6-Diacetyl-3, 6-diferuloylsucrose | 19 | |
| 8-Oxo-pinoresinol | 20 | |
| 13-Hydroxy helianthemum 8-(17), 14-diene-19-aldehyde | 22 | |
| (24S)-24-Ethylcholesta-3β, 5α, 6α-triol | 23 | |
| Cucurbitacin Ⅱa | 23 | |
| Cucurbitacin U | 23 | |
| Asteryunnanoside F | 23 | |
| Saikosaponin M | 23 | |
| Guanyeliaoine Ⅰ | 45 | |
| Guanyeliaoine Ⅱ | 45 | |
| Polygonum pubescens Blume. (Fu Mao Liao, 伏毛蓼) |
Vanicoside A′ (121) | 46 |
| Hydropiperoside-B (122) | 46 | |
| Hydropiperoside A (123) | 46 | |
| Polygonum runcinatum (Yu Ye Liao, 羽叶蓼) |
3, 3, 4′-Trimethylellagic acid | 41 |
| 3, 3-Di-O-methyl ellagic acid | 41 | |
| 3, 3, 4-Tri-O-methylellagic acid -4′-d-glucopyranoside | 41 | |
| Runcinatside | 38 | |
| 3, 3′-Dimethylellagic acid | 38 | |
| 3, 3′, 4′-Trimethylellagic acid | 38 | |
| 3, 3′-Dimethylellagic acid-4′-O-β-d-glucoside | 38 | |
| 3-Methylellagic acid-4′-O-a-l-rhamno-pyranoside | 38 | |
| Polygonum sieboldii (Jian Ye Liao, 箭叶蓼) |
Tricin | 5 |
| 7, 4-Dimethoxy kaempferol | 5 | |
| Polygonum viscosum (Xiang Liao, 香蓼) |
Iscozulenic acid methylester | 26 |
| Viscoazucinic acid | 26 | |
| Polygosumic acid | 26 | |
| Viscosumic acid | 28 | |
| Polygonum viviparum L. (Zhu Ya Liao, 珠芽蓼) |
Viviparum A | 14 |
| Viviparum B | 14 | |
| Rumex japonicus (Yang Ti, 羊蹄) |
Rhein | 13 |
| Emodin-6-methyl-ether | 13 |
In recent years, studies have shown that the genus Polygonum (Polygonaceae) possesses good anticancer and antitumor activities. Moreover, P. cuspidatum has antiviral effects on human immunodeficiency virus [47] and anticancer effects in lung cancer [48], hepatic carcinoma [49-53], colorectal cancer [54], and oral cancer [55]. Intisar [56] isolated 13 phenolic compounds from methanol-water extracts of P. bistorta L. using gas chromatography-mass spectrometry and liquid chromatography with photodiode-array detection and tandem electrospray ionization mass spectrometry, and these compounds were found to show strong cytotoxicity against HCCLM3 cancer cells. Additionally, all the fractions containing phenolic content showed good to strong cytotoxic activity. Manoharan [57] evaluated the cytotoxic activities of P. bistorta against P338, HepG2, J82, HL60 (human leukemia), MCF7, and LL2 cancer cell lines and showed that the chloroform fractions exhibited good activity against P388, HL60, and LL2 cancer cell lines.
Dai [58] used 3H-TdR incorporation for drug susceptibility testing and found that extracts of P. cuspidatum possessed good inhibitory effects on HepG2 cancer cells. In addition, resveratrol and its glycosides isolated from Rumex gmelini inhibited MCF-7 breast cancer cell proliferation [59] and had obvious inhibitory effects on human and mouse white blood cell hyperplasia [60]. Sun [61] determined the inhibitory effects of petroleum ether extracts of P. orientale (mainly containing alkenes, alkyne hydrocarbons, and esters) on SPAC-1 lung cancer cells, U87 glioma cells, HeLa cervical cancer cells, SGC7901 gastric cancer cells, and KB oral epidermoid carcinoma cells by MTT assays; the extract was found to have inhibitory effects on HeLa and SGC7901 cells.
Most species of the genus Polygonum (Polygonaceae) have antioxidant effects. P. cuspidatum is a potent anti-oxidative traditional Chinese medicine [62, 63]. Stilbenes and anthraquinones (2-methoxy-6-acetyl-7- methyljuglone) [64] are key compounds possessing antioxidant effects [65]. In addition, (+) - catechin and (-) - epicatechin also showed high radical scavenging activities in DPPH radical scavenging assays [66].
Ahmad [67] and George [68] found that P. minus showed the highest DPPH radical scavenging activity. Additionally, Xing [69] investigated the antioxidant and α-glucosidase inhibitory activities of P. perfoliatum L. and found that the methanol extracts had strong antioxidant and α-glucosidase inhibitory activities. Chang [70] studied the methanol extracts of P. perfoliatum and evaluated the antioxidant activities of the isolated compounds. Among them, α-tocopherol and methyl trans-ferulate showed significant DPPH free radical scavenging effects, with EC50 values of 11.9 μg/mL and 7.8 μg/mL, respectively. Wang [71] found that the ethanol extracts of runcinate knotweed had strong antioxidant effects. Moreover, Ayaz [72] showed that P. hydropiper was enriched with potent bioactive compounds, which could be used for the treatment of various neurological disorders. Hsu [73] studied the anti-oxidative activities of P. aviculare L. by free radical scavenging assays, superoxide radical scavenging assays, lipid peroxidation assays, and hydroxyl radical-induced DNA strand scission assays strand scission assays and showed that P. aviculare L. extract had strong antioxidant effects.
Studies have shown that flavonoids from the genus Polygonum (Polygonaceae) have significant anti-inflammatory and analgesic effects. In particular, luteolin has been shown to have potent anti-inflammatory and analgesic effects, potentially through inhibition of the release of inflammatory mediators, such as prostaglandin E2 (PGE2), and suppression of nuclear factor-κB-mediated gene expression [74]. In addition, kaempferol and quercetin inhibit the synthesis of inflammatory mediators, such as PGE2, by blocking the expression of cyclooxygenase-2 to relieve inflammation [75-78].
George [79] found that P. minus possesses potent anti-inflammatory activities, and Song [80] studied the antibacterial and anti-inflammatory effects of Caulis Polygoni Multiflori (Ye Jiao Teng, 夜交藤). The results showed that Caulis Polygoni Multiflori (Ye Jiao Teng, 夜交藤) had strong anti-inflammatory effects on chronic inflammation and no acute inflammation resistance effect. Fan [81] found that quercetin-3-O-β-d-glucuronide showed inhibitory activity against influenza A virus. Additionally, Rahman [82] studied the analgesic activities of the hexane, ethylacetate, and methanol extracts of P. hydropiper using the acetic acid-induced writhing assays. The results showed significant effects on acetic acid-induced writhing. Among the tested extracts, the ethylacetate extract showed the most significant activity.
P. capitatum exhibits significant anti-inflammatory activities [83-86]. In particular, stilbenes and hydroxyanthraquinones show a broad antibacterial range [87-89]. Using the paper disc diffusion method, researchers showed that P. aviculare L. exhibits good activity against both Gram-negative and Gram- positive bacteria and fungi [90] and that P. glabraum possesses significant antimicrobial activity [91]. ElHaci [92] studied the biological activities of P. maritimum L. from the Algerian coast and found that its activity (methanolic crude extract of P. maritimum; PMCE) was probably due to phenolic compounds present in the extract because PMCE had a very high content of total phenol and showed high anti-bacterial activity against gram-positive bacterial strains (e.g., Bacillus cereus, B. subtilis, and Staphylococcus aureus). Additionally, Majumder [93] investigated the anthelmintic and cytotoxic activities of crude methanolic extracts of P. viscosum and showed that the crude methanolic extract of P. viscosum leaves possessed significant, dose-dependent anthelmintic activity; the activity of the crude extract was comparable to that of standard drugs.
Polydatin from P. cuspidatum and P. aviculare L. shows anti-obesity effects in obese mice fed a high-fat diet [94-96] and on lipid profiles in hyperlipidemic rabbits [97]. In addition, this compound had inhibitory effects on Coxsackievirus B4 [98] and showed wound healing in rats [99] and has been identified as an inhibitor of the bacterial DNA primase enzyme [100]. Studies have shown that trans-resveratrol from P. cuspidatum and the combined extracts of Morus alba and P. odoratum leaves play protective roles against bone loss [101, 102]. Finally, Datta [103] isolated eight compounds from P. viscosum and showed that these compounds had good inhibitory effects on the central nervous system.
Plants of the genus Polygonum (Polygonaceae) produce a range of chemical constituents with various pharmacological effects. To date, only some of the species in this genus have been explored. Only 123 compounds have been reported, most of which have not been evaluated to determine their pharmacological effects. Some extracts have showed potential efficacy; for example, extracts from Polygonum species possess anticancer, antitumor, anti-oxidative, anti-inflammatory, analgesic, antimicrobial, and insecticidal effects. Therefore, the genus Polygonum should be investigated and explored further for identification of new drug candidates.
We thank for the funding support from the National Natural Science Foundation of China (No. 81374062 and No. 81673579) and Hunan Province University Innovation Platform Open Fund (Project 13K077).
The authors declare no conflict of interest.
| [1] |
HOU K Z. The dictionary of genera of seed plants Chinese. Beijing: Science Press, 1982: 291.
|
| [2] |
MAN-MAN L I, LIU Z H, WANG H Y, et al. Studies on the antibacterial activities and chemical constituents of Polygonum aviculare L. Natural Product Research & Development, 2014, 26(4):526-530. https://www.sciencedirect.com/science/article/pii/S1319562X09000539
|
| [3] |
YUNUSKHODZHAEVA N A, ESHBAKOVA K A, ABDULLABEKOVA V N. Flavonoid composition of the herb Polygonum aviculare.Chemistry of Natural Compounds, 2010, 46(5):803-804. doi: 10.1007/s10600-010-9749-4
|
| [4] |
LIN Y, ZHANG C F, ZHANG M. Study on the flavonoids of Polygonum jucundum. China Journal of Chinese Materia Medica, 2009, 34(6), 1690-1691. https://www.researchgate.net/publication/248225885_Flavonoids_from_Polygonum_minus
|
| [5] |
PENG L, ZHANG C, Xu X. Studies on the chemical constituents from Polygonum sieboldii. Pharmaceutical and Clinical Research, 2013, 21(5), 347-349. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jsyxylcyj201304012
|
| [6] |
PARTOVI T, ZABIHI M. Coagulant compounds from Rhizomes of Polygonum bistorta. Chemistry of Natural Compounds, 2014, 50(1):126-127. doi: 10.1007/s10600-014-0885-0
|
| [7] |
KURKINAA V, RYAZANOVAT K, KURKINV A. Flavonoids from the Aerial Part of Polygonum persicaria. Chemistry of Natural Compounds, 2013, 49(5):845-847. doi: 10.1007/s10600-013-0761-3
|
| [8] |
TANTRYM A, RAHMANA A. Amplexicine, an antioxidant flavan-3-ol from Polygonum amplexicaule. Natural Product Communications, 2011, 6(11):1597-1598. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0225795597/
|
| [9] |
WANG K J, ZHANG Y J, YANG C R. Antioxidant phenolic compounds from rhizomes of Polygonum paleaceum. Journal of Ethnopharmacology, 2005, 96(3):483. doi: 10.1016/j.jep.2004.09.036
|
| [10] |
NIKOLAEVAG G, LAVRENTM V, NIKOLAEVAI G. Phenolic compounds from several Polygonum, species. Chemistry of Natural Compounds, 2009, 45(5):735-736. doi: 10.1007/s10600-009-9414-y
|
| [11] |
LI X, YU M, MENG D, et al. A new chromone glycoside from Polygonum capitatum. Fitoterapia, 2007, 78(7-8):506-509. doi: 10.1016/j.fitote.2007.05.003
|
| [12] |
LI F, ZHAN Z, LIU F, et al. Polyflavanostilbene A, a new flavanol-fused stilbene glycoside from Polygonum cuspidatum. Organic Letters, 2013, 15(3):674-677. doi: 10.1021/ol3035033
|
| [13] |
MORADI-AFRAPOLI F, ASGHARI B, SAEIDNIA S, et al. In vitro, α-glucosidase inhibitory activity of phenolic constituents from aerial parts of Polygonum hyrcanicum. Daru Journal of Pharmaceutical Sciences, 2012, 20(1):37. doi: 10.1186/2008-2231-20-37
|
| [14] |
ZHENG S Z, LI K L, WANG J X, et al. Two new flavone glycosides from Polygonum viviparum L. Indian Journal of Chemistry, 2001, 40(2):167-169. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ029665726/
|
| [15] |
LIU J, ZHANG Q, CHEN K, et al. Small-molecule STAT3 signaling pathway modulators from Polygonum cuspidatum. Planta Medica, 2012, 78(14):1568. doi: 10.1055/s-00000058
|
| [16] |
GUO S, FENG B, ZHU R, et al. Preparative isolation of three anthraquinones from Rumex japonicus by high-speed counter-current chromatography. Molecules, 2011, 16(2):1201. doi: 10.3390/molecules16021201
|
| [17] |
HAQUE S N. A New naphthoquinone from Polygonum Aviculare. Natural Product Letters, 2002, 16(2):115. doi: 10.1080/10575630290020019
|
| [18] |
ZHANG H, ZHANG Q W, WANG L, et al. Two new anthraquinone malonylglucosides from Polygonum cuspidatum. Natural Product Research, 2012, 26(14):1323. doi: 10.1080/14786419.2011.578072
|
| [19] |
SUN X, ZIMMERMANNM L, Campagne J M, et al. New sucrose phenylpropanoid esters from Polygonum perfoliatum. Journal of Natural Products, 2000, 63(8):1094. doi: 10.1021/np000055e
|
| [20] |
WANG K W, ZHU J R, SHEN L Q. A new lignan with anti-tumour activity from Polygonum perfoliatum L. Natural Product Research, 2013, 27(6):568-573. doi: 10.1080/14786419.2012.682993
|
| [21] |
XIAO K, XUAN L, XU Y, et al. Constituents from Polygonum cuspidatum. Chemical & Pharmaceutical Bulletin, 2002, 50(5):605-608. http://d.old.wanfangdata.com.cn/Periodical/shzyyzz200808033
|
| [22] |
CHENG H B, LIU X Q, CHEN K L. Chemical constituents of ethyl acetate extract from Polygonum perfoliatum. Journal of Chinese Medicinal Materials, 2012, 35(7):1088. http://d.old.wanfangdata.com.cn/Periodical/zyc201207021
|
| [23] |
LI H F, MA Q Y, LIU Y, et al. Chemical constituents from Polygonum perfoliatum. Chinese Journal of Applied & Environmental Biology, 2009, 15(5):615-620. http://d.old.wanfangdata.com.cn/Periodical/yyyhjswxb200905007
|
| [24] |
SUN X B, ZHAO P H, XU Y J, et al. Chemical constituents from the roots of Polygonum bistorta. Chemistry of Natural Compounds, 2007, 43(5):563-566. doi: 10.1007/s10600-007-0193-z
|
| [25] |
MAZID M A, DATTA B K, NAHAR L, et al. Phytochemical Studies on Polygonum barbatum (L.) Hara var. barbata (Polygonaceae). Records of Natural Products, 2011, 5(2):143-146. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_55ee2a4f31532b219bf0d4d793add72c
|
| [26] |
DATTAB K, RAHMANM M, GRAYA I, et al. Polygosumic acid, a new cadinane sesquiterpene from Polygonum viscosum, inhibits the growth of drug-resistant Escherichia coli, and Staphylococcus aureus, (MRSA) in vitro. Journal of Natural Medicines, 2007, 61(4):391-396. doi: 10.1007/s11418-007-0165-4
|
| [27] |
YANG Z Y, QIAN S H, QIN M J. A new triterpenoid saponin from the fruits of Polygonum orientale. Yao Xue Xue Bao, 2008, 43(4), 388-391. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yxxb200804011
|
| [28] |
DATTA B K, DATTA S K, RASHID M A, et al. A sesquiterpene acid and flavonoids from Polygonum viscosum. Phytochemistry, 2000, 54(2):201. doi: 10.1016/S0031-9422(00)00057-1
|
| [29] |
MANOHARAN K P, BENNYT K, YANG D. Cycloartane type triterpenoids from the rhizomes of Polygonum bistorta. Phytochemistry, 2005, 66(19):2304-2308. doi: 10.1016/j.phytochem.2005.07.008
|
| [30] |
KAI X, XUAN L, XU A, et al. Stilbene glycoside sulfates from Polygonum cuspidatum. Journal of Natural Products, 2000, 63(10):1373-1376. doi: 10.1021/np000086+
|
| [31] |
LI S G, CHEN L L, HUANG X J, et al. Five new stilbene glycosides from the roots of Polygonum multiflorum. Journal of Asian Natural Products Research, 2013, 15(11):1145-1151. doi: 10.1080/10286020.2013.837454
|
| [32] |
XIANG M, SU H, HU J, et al. Isolation, identification and determination of methyl caffeate, ethyl caffeate and other phenolic compounds from Polygonum amplexicaule var. sinense. Journal of Medicinal Plant Research, 2011, 5(9):1685-1691. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=Open J-Gate000003823572
|
| [33] |
TAKASAKI M, KUROKI S, KOZUKA M, et al. New phenylpropanoid esters of sucrose from Polygonum lapathifolium. Journal of Natural Products, 2001, 64(10):1305-1308. doi: 10.1021/np010222q
|
| [34] |
TAKASAKI M, KONOSHIMA T, KUROKI S, et al. Cancer chemopreventive activity of phenylpropanoid esters of sucrose, vanicoside B and lapathoside A, from Polygonum lapathifolium. Cancer Letters, 2001, 173(2):133. doi: 10.1016/S0304-3835(01)00670-X
|
| [35] |
LIU X Q, HUA H M, LIU J, et al. A new tannin-related compound from the rhizome of Polygonum bistorta L.. Journal of Asian Natural Products Research, 2006, 8(4):299. doi: 10.1080/10286020500034956
|
| [36] |
YONG-JUNL I, XUN H E, LIU Z B, et al. Chemical constituents of flowers of Polygonum orientale. Lishizhen Medicine & Materia Medica Research, 2010, 21(1):14-15. http://d.old.wanfangdata.com.cn/Periodical/zgzyzz200920014
|
| [37] |
SMOLARZH D, POTRZEBOWSKIM J. Persilben, a new carboxystilbene from Polygonum persicaria. Journal of Molecular Structure, 2002, 605(2-3):151-156. doi: 10.1016/S0022-2860(01)00758-X
|
| [38] |
ZHOU Z H, LIU M Z, WANG M H, et al. A new ellagic acid derivative from Polygonum runcinatum. Natural Product Research, 2015, 29(9):795-799. doi: 10.1080/14786419.2014.986727
|
| [39] |
YANG Y X, AN M M, JIN Y S, et al. Chemical constituents from the rhizome of Polygonum paleaceum and their antifungal activity. Journal of Asian Natural Products Research, 2017, 19(1):47-52. doi: 10.1080/10286020.2016.1196672
|
| [40] |
SAID M S, CHINCHANSURE A A, NAWALE L, et al. A new butenolide cinnamate and other biological active chemical constituents from Polygonum glabrum. Natural Product Research, 2015, 29(22):2080-2086. doi: 10.1080/14786419.2015.1004674
|
| [41] |
WANG L N, XU B X, CAO P X, et al. Studies on the chemical constituents of Polygonum runcinatum Buch.-Ham.var.sinense Hemsl. Natural Product Research & Development, 2009.21(1):73. http://en.cnki.com.cn/Article_en/CJFDTOTAL-TRCW200901018.htm
|
| [42] |
HU H B, WANG G W, LIU J X, et al. Studies on phenolic compounds from Polygomun aviculane. China Journal of Chinese Materia Medica, 2006, 31(9):740-742. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgzyzz200609010
|
| [43] |
ZHANG L J, WANG Y L, WANG Z, et al. Study on the chemical constituents of the active fraction of Polygonum capitatum. Journal of Chinese Medicinal Materials, 2012, 35(9):1425-1428. http://d.old.wanfangdata.com.cn/Periodical/zyc201209016
|
| [44] |
ZHANG G. Y, ZENG T. Study on chemical ingredients of Polygonum hydropiper L. Chemistry and Industry of Forest Products, 2005, 25(3), 21-24. https://www.researchgate.net/publication/298264640_Study_on_chemical_constituents_of_Polygonum_hydropiper_Linn
|
| [45] |
SHEN T, JIA Z J, ZHENG S Z. Studies on chemical constituents of Polygonum perforliatum L. Journal of Asian Natural Products Research, 2007, 9(2):129. doi: 10.1080/1028602042000324844
|
| [46] |
LIU Q, LI B, ZHAO J, et al. A new sucrosephenylpropanoid ester from Polygonum pubescens Blume. Natural Product Research, 2017, 31(15):1725. doi: 10.1080/14786419.2017.1289208
|
| [47] |
LIN H W, SUN M X, WANG Y H, et al. Anti-HIV activities of the compounds isolated from Polygonum cuspidatum and Polygonum multiflorum. Planta Medica, 2010, 76(9):889-892. doi: 10.1055/s-0029-1240796
|
| [48] |
LIN Y W, YANG F J, CHEN C L, et al. Free radical scavenging activity and antiproliferative potential of Polygonum cuspidatum root extracts. Journal of Natural Medicines, 2010, 64(2):146-152. doi: 10.1007/s11418-009-0387-8
|
| [49] |
HU B, ANHM, SHENK P, et al. Polygonum cuspidatum extract induces anoikis in hepatocarcinoma cells associated with generation of reactive oxygen species and downregulation of focal adhesion kinase. Evidence-based complementary and alternative medicine : eCAM, 2012, 2012(4):607-675. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3449140
|
| [50] |
JUNG K A, MIN H J, YOO S S, et al. Drug-induced liver injury: twenty five cases of acute hepatitis following ingestion of Polygonum multiflorum Thunb. Gut & Liver, 2011, 5(4):493-499. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3240794
|
| [51] |
MOHD GHAZALIM A, AL-NAQEB G, KRISHNANS K, et al. Apoptosis Induction by Polygonum minus is related to antioxidant capacity, alterations in expression of apoptotic-related genes, and S-phase cell cycle arrest in HepG2 cell line. Biomed Research International, 2014, 2014:539-607. https://www.hindawi.com/journals/bmri/2014/539607/
|
| [52] |
XIE Q, YANGY, WANG Z, et al. Resveratrol-4-O-D-(2'-galloyl)-glucopyranoside isolated from Polygonum cuspidatum Exhibits anti-hepatocellular carcinoma viability by inducing apoptosis via the JNK and ERK pathway. Molecules, 2014, 19(2):1592. doi: 10.3390/molecules19021592
|
| [53] |
SAVOURETJ F, QUESNEM. Resveratrol and cancer: a review. Biomedicine & Pharmacotherapy, 2002, 56(2):84-87. http://d.old.wanfangdata.com.cn/Periodical/lsyyz200611014
|
| [54] |
JI Q, LIU X, FU X, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. Plos One, 2013, 8(11):787. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0078700
|
| [55] |
SHIN J A, SHIM J H, JEON J G, et al. Apoptotic effect of Polygonum Cuspidatum in oral cancer cells through the regulation of specificity protein 1. Oral Diseases, 2011, 17(2):162. doi: 10.1111/j.1601-0825.2010.01710.x
|
| [56] |
INTISARA, ZHANG L, LUO H, et al. Anticancer constituents and cytotoxic activity of methanol-water extract of Polygonum bistorta L. African Journal of Traditional Complementary & Alternative Medi, 2013, 10(1):53-59. http://europepmc.org/articles/PMC3746358
|
| [57] |
MANOHARANK P, YANG D, HSU A, et al. Evaluation of Polygonum bistorta for anticancer potential using selected cancer cell lines. Medicinal Chemistry, 2007, 3(2):121-126. doi: 10.2174/157340607780059495
|
| [58] |
DAI G H, YANG F, TONG H L, et al. Experimental study of Polygonum cuspidatum extract against human hepatocellular carcinoma cell line HepG-2. Chinese Journal of Traditional Medical Science and Technology, 2009, 16, 375-377. https://www.researchgate.net/publication/235750205_Synergistic_anticancer_effects_of_curcumin_and_resveratrol_in_Hepa1-6_hepatocellular_carcinoma_cells
|
| [59] |
SCHNEIDER Y, VINCENT F, DURANTON B, et al. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Letters, 2000, 158(1):85-91. doi: 10.1016/S0304-3835(00)00511-5
|
| [60] |
FILIPV, PLOCKOVA M, ŠMIDRKALJ, et al. Resveratrol and its antioxidant and antimicrobial effectiveness. Food Chemistry, 2003, 83(4):585-593. doi: 10.1016/S0308-8146(03)00157-2
|
| [61] |
SUN F P, LOU Y C, ZHANG D Q, et al. Anti-tumor study from the petroleum ether extract of Polygonum orientale. Chinese Traditional Patent Medicine, 2012, 34, 938-940. doi: 10.3389/fphar.2017.00562/full
|
| [62] |
LEE C C, CHEN Y T, CHIU C C, et al. Polygonum cuspidatum, extracts as bioactive antioxidaion, anti-tyrosinase, immune stimulation and anticancer agents. Journal of Bioscience & Bioengineering, 2015, 119(4):464-469. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0234907029
|
| [63] |
HSU C Y, CHAN Y P, CHANG J. Antioxidant activity of extract from Polygonum cuspidatum. Biological Research, 2007, 40(1):13-21. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=Open J-Gate000000265398
|
| [64] |
LI Y B, LIN Z Q, ZHANG Z J, et al. Protective, antioxidative and antiapoptotic effects of 2-methoxy-6-acetyl-7-methyljuglone from Polygonum cuspidatum in PC12 cells. Planta Medica, 2011, 77(4):354-361. doi: 10.1055/s-0030-1250385
|
| [65] |
OKANO T, MIYAKAWA K. Key compound groups for the neuroprotective effect of roots of Polygonum cuspidatum on transient middle cerebral artery occlusion in Sprague-Dawley rats. Natural Product Research, 2010, 24(13):1214-1226. doi: 10.1080/14786410902992157
|
| [66] |
WENS, BAO J, WEI L, et al. 2-Methoxy-6-acetyl-7-methyljuglone (MAM), a natural naphthoquinone, induces NO-dependent apoptosis and necroptosis by H2O2-dependent JNK activation in cancer cells. Free Radical Biology & Medicine, 2016, 92: 61-77. https://www.sciencedirect.com/science/article/pii/S0891584916000253
|
| [67] |
AHMAD R, BAHARUMS N, BUNAWANH, et al. Volatile profiling of aromatic traditional medicinal plant, Polygonum minus in different tissues and its biological activities. Molecules, 2014, 19(11):19220-19242. doi: 10.3390/molecules191119220
|
| [68] |
GEORGEA, NG C P, O'CALLAGHANM, et al. In vitro and ex-vivo cellular antioxidant protection and cognitive enhancing effects of an extract of Polygonum minus Huds demonstrated in a Barnes Maze animal model for memory and learning. BMC Complementary & Alternative Medicine, 2014, 14(1):161. doi: 10.1186/1472-6882-14-161
|
| [69] |
XING Y J, WANG H Y, WANG J X, et al. α-glucosidase inhibitory and antioxidant activities of Polygonum perfoliatum. Chinese Journal of Experimental Traditional Medical Formulae, 2011, 17(2):189-191. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgsyfjxzz201102054
|
| [70] |
CHANG C, FEIJANE T, CHANGHUNG C. Natural products from Polygonum perfoliatum and their diverse biological activities. Natural Product Communications, 2008, 3(9):1385-1386. http://www.cabdirect.org/abstracts/20083296518.html
|
| [71] |
WANG X Y. Study on antioxidation of ethanol extract from Runcinate Knotweed Herb. Journal of Jinggangshan University, 2010, 31, 58-64. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jgsxyxb201003012
|
| [72] |
AYAZ M, JUNAID M, AHMED J, et al. Phenolic contents, antioxidant and anticholinesterase potentials of crude extract, subsequent fractions and crude saponins from Polygonum hydropiper L. BMC Complementary and Alternative Medicine, 2014, 14(1):145. doi: 10.1186/1472-6882-14-145
|
| [73] |
HSU C Y. Antioxidant activity of extract from Polygonum aviculare L. Biological Research, 2006, 39(2):281. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=Open J-Gate000000265397
|
| [74] |
WANG X G, CHEN G Y, CHEN M P. Effect of luteolin on COX-2 and mPGES-1 expression in LPS-induced RAW264.7 cells. Zhong Yao Cai, 2007, 30(30):1263-1266. http://www.ncbi.nlm.nih.gov/pubmed/18300499
|
| [75] |
MILES E A, ZOUBOULI P, CALDER P C. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition, 2005, 21(3):389-394. doi: 10.1016/j.nut.2004.06.031
|
| [76] |
O'LEARY K A, DEP S, DE P S, et al. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription.. Mutation Research/fundamental & Molecular Mechanisms of Mutagenesis, 2004, 551(1/2):245-254. http://www.ncbi.nlm.nih.gov/pubmed/15225597
|
| [77] |
ABD EL-KADERA M, EL-READIM Z, AHMEDA S, et al. Polyphenols from aerial parts of Polygonum bellardii and their biological activities. Pharmaceutical Biology, 2013, 51(8):1026-1034. doi: 10.3109/13880209.2013.775160
|
| [78] |
BRALLEY E E, GREENSPAN P, HARGROVE J L, et al. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. Journal of Inflammation, 2008, 5(1):1. doi: 10.1186/1476-9255-5-1
|
| [79] |
GEORGE A, CHINNAPPAN S, CHINTAMANENI M, et al. Anti-inflammatory effects of Polygonum minus (Huds), extract in in-vitro enzyme assays and carrageenan induced paw edema. BMC Complementary and Alternative Medicine, 2014, 14(1):355. doi: 10.1186/1472-6882-14-355
|
| [80] |
SONG Y, TANG R, ZHANG Z Y. Anti -inflammatory and bacteria inhibition effects of caulis Polygoni multiflri. West China Journal of Pharmaceutical Sciences, 2003, 18, 112-114.
|
| [81] |
FAN D, ZHOU X, ZHAO C, et al. Anti-inflammatory, antiviral and quantitative study of quercetin-3-O-β-D-glucuronide in Polygonum perfoliatum L. Fitoterapia, 2011, 82(6):805-810. doi: 10.1016/j.fitote.2011.04.007
|
| [82] |
RAHMAN E, GONI S A, RAHMANM T, et al. Antinociceptive activity of Polygonum hydropiper.Fitoterapia, 2002, 73(7-8):704-706. doi: 10.1016/S0367-326X(02)00239-3
|
| [83] |
SHAN B, CAI Y Z, JOHAD B, et al. Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chemistry, 2008, 109(3):530-537. doi: 10.1016/j.foodchem.2007.12.064
|
| [84] |
KWON Y R, SON K J, PANDITS, et al. Bioactivity-guided separation of anti-acidogenic substances against Streptococcus mutans UA 159 from Polygonum cuspidatum. Oral Diseases, 2010, 16(2):204-209. doi: 10.1111/odi.2010.16.issue-2
|
| [85] |
PANDIT S, KIM H J, PARK S H, et al. Enhancement of fluoride activity against Streptococcus mutans biofilms by a substance separated from Polygonum cuspidatum. Biofouling, 2012, 28(3):279-287. doi: 10.1080/08927014.2012.672646
|
| [86] |
LIAO S G, ZHANG L J, SUN F, et al. Antibacterial and anti-inflammatory effects of extracts and fractions from Polygonum capitatum. Journal of Ethnopharmacology, 2011, 134(3):1006. doi: 10.1016/j.jep.2011.01.050
|
| [87] |
SONG J H, KIM S K, CHANG K W, et al. In vitro inhibitory effects of Polygonum cuspidatum on bacterial viability and virulence factors of Streptococcus mutans and Streptococcus sobrinus. Archives of Oral Biology, 2006, 51(12):1131-1140. doi: 10.1016/j.archoralbio.2006.06.011
|
| [88] |
KIM Y S, HWANG C S, SHIN D H. Volatile constituents from the leaves of Polygonum cuspidatum S. et Z. and their anti-bacterial activities. Food Microbiology, 2005, 22(1):139-144. doi: 10.1016/j.fm.2004.01.016
|
| [89] |
BAN S H, YOUNGRAN K, PANDIT S, et al. Effects of a bio-assay guided fraction from Polygonum cuspidatum root on the viability, acid production and glucosyltranferase of mutans streptococci. Fitoterapia, 2010, 81(1):30. doi: 10.1016/j.fitote.2009.06.019
|
| [90] |
SALAMAH M H, MARRAIKI N. Antimicrobial activity and phytochemical analyses of Polygonum aviculare L. (Polygonaceae), naturally growing in Egypt. Saudi Journal of Biological Sciences, 2010, 17(1):57. doi: 10.1016/j.sjbs.2009.12.009
|
| [91] |
MEERA R, RAPHEL J, DEVI P, et al. Analgesic and anti microbial activity of aerial parts of Polygonum glabraum. International Journal of Pharmaceutical Research & Allied Sciences, 2013, 2(4):38-41. http://connection.ebscohost.com/c/articles/93465354/analgesic-anti-microbial-activity-aerial-parts-polygonum-glabraum
|
| [92] |
EL-HACI I A, BEKKARAF A, MAZARIW, et al. Screening of biological activities of Polygonum maritimum L. from Algerian coast. Asian Pacific Journal of Tropical Biomedicine, 2013, 3(8):611-616. doi: 10.1016/S2221-1691(13)60124-0
|
| [93] |
MAJUMDERM S, AMIN M N, MOGHALM M R, et al. Anthelmintic and cytotoxic activities of two medicinal plants: Polygonum viscosum and Aphanamixis polystachya Growing in Bangladesh. Journal of Scientific Research, 2014, 6(2):339-345. https://www.researchgate.net/publication/262912402_Anthelmintic_and_Cytotoxic_Activities_of_Two_Medicinal_Plants_Polygonum_viscosum_and_Aphanamixis_polystachya_Growing_in_Bangladesh
|
| [94] |
JIAN D, SUN L N, XING W W, et al. Lipid-lowering effects of polydatin from Polygonum cuspidatum in hyperlipidemic hamsters. Phytomedicine International Journal of Phytotherapy & Phytopharmacology, 2009, 16(6-7):652-658. doi: 10.1016-j.phymed.2008.10.001/
|
| [95] |
KUO C H, CHEN B Y, LIU Y C, et al. Optimized ultrasound-assisted extraction of phenolic compounds from Polygonum cuspidatum. Molecules, 2014, 19(1):67-77. http://www.mdpi.com/1420-3049/19/1/67
|
| [96] |
HUI-CHUN X, HONG-PING H, ZHI C, et al. A study on the effect of resveratrol on lipid metabolism in hyperlipidemic mice. African Journal of Traditional Complementary & Alternative Medicines Ajtcam, 2013, 11(1):209. http://www.ncbi.nlm.nih.gov/pubmed/24653579
|
| [97] |
XING W W, WU J Z, MIN J, et al. Effects of polydatin from Polygonum cuspidatum on lipid profile in hyperlipidemic rabbits.. Biomedicine & Pharmacotherapy, 2009, 63(7):457-462. https://www.sciencedirect.com/science/article/pii/S0753332208001637
|
| [98] |
LIU Z, WEI F, CHEN L J, et al. In vitro and in vivo studies of the inhibitory effects of emodin isolated from Polygonum cuspidatum on Coxsakievirus B4. Molecules, 2013, 18(10):11842-11858. doi: 10.3390/molecules181011842
|
| [99] |
WU X B, LUO X Q, GU S Y, et al. The effects of Polygonum cuspidatum extract on wound healing in rats. Journal of Ethnopharmacology, 2012, 141(3):934. doi: 10.1016/j.jep.2012.03.040
|
| [100] |
HEGDE V R, PU H, PATELM, et al. Two new bacterial DNA primase inhibitors from the plant Polygonum cuspidatum. Bioorganic & Medicinal Chemistry Letters, 2004, 35(36):2275-2277. https://www.sciencedirect.com/science/article/pii/S0960894X04002094
|
| [101] |
LIU Z P, LI W X, YU B, et al. Effects of trans-resveratrol from Polygonum cuspidatum on bone loss using the ovariectomized rat model. Journal of Medicinal Food, 2005, 8(1):14. doi: 10.1089/jmf.2005.8.14
|
| [102] |
SUNGKAMANEE S, WATTANATHORN J, MUCHIMAPURAS, et al. Antiosteoporotic Effect of Combined Extract of Morus alba and Polygonum odoratum. Oxidative Medicine & Cellular Longevity, 2014, 2014(3):579305-579305. https://www.hindawi.com/journals/omcl/2014/579305/
|
| [103] |
DATTA B K, DATTA S K, CHOWDHURYM M, et al. Analgesic, antiinflammatory and CNS depressant activities of sesquiterpenes and a flavonoid glycoside from Polygonum viscosum. Pharmazie, 2004, 59(3):222. https://www.researchgate.net/publication/8626450_Analgesic_Antiinflammatory_and_CNS_Depressant_Activities_of_Sesquiterpenes_I-III_and_a_Flavonoid_Glycoside_IV_from_Polygonum_viscosum
|
| Plant | Compounds | Reference |
| Polygonum aviculare L. (Bian Xu, 扁蓄) |
Kaempferol | 2 |
| Myricetin | 2 | |
| Myricetin3-O-(3" -O-galloy1)-rhamnopyranoside | 2 | |
| Liquiritin | 3 | |
| Avicularin | 3 | |
| Cinaroside | 3 | |
| 6-Methoxyplumbagin | 17 | |
| Polygonum amplexicaule (Bao Jing Liao, 抱茎蓼) |
(2R, 3S)-3′, 4′, 5, 6, 7-pentahydroxyflavan-3-ol | 8 |
| Polygonum angustifolium (Xi Ye Liao, 细叶蓼) |
Avicularin | 10 |
| Polygonum amphibium (Liang Xi Liao, 两栖蓼) |
Hyperoside | 10 |
| Polygonum aviculane (Bian Xu Liao, 扁蓄蓼) |
Rosemary acid | 42 |
| Caffeic acid | 42 | |
| Coumaric acid | 42 | |
| Polygonum bistorta L. (Quan Liao, 拳蓼) |
3, 7, 3-Trihydroxy-5, 6-dimethoxyflavone | 6 |
| 3β-Acetoxy-dammara-20, 24-diene | 24 | |
| Arborinone | 24 | |
| Adianenone | 24 | |
| Arborinol | 24 | |
| 24(E)-ethylidenecycloartanone | 29 | |
| 24(E)-ethylidenecycloartan-3α-ol | 29 | |
| 3-Methyl-gallic-acid, 4-O-β-d-(6′-O-3" -methyl-galloyl)-glucopyranoside | 35 | |
| 3-Methyl-gallic-acid, 4-O-β-d-(6′-O-3" -methyl-galloyl)-glucopyranoside | 35 | |
| Polygonum barbatum L. (Mao Liao, 毛蓼) |
Sitosterone | 25 |
| Viscozulenic acid | 25 | |
| Polygonum capitatum (Tou Hua Liao, 头花蓼) |
7-O-(6′-galloyl)-β-d-glucopyranosyl-5-hydroxychromone | 11 |
| Hirsutine | 43 | |
| Polygonum cuspidatum (Hu Zhang, 虎杖) |
Polyganins A | 18 |
| Polyganins B | 18 | |
| Polyflavanostilbene A | 12 | |
| Sodium(-)-lyoniresinol-2α-sulfate | 21 | |
| Sodium(+)-isolaricireinol-2α-sulfate | 21 | |
| Polygonum divaricatum (Cha Fen Liao, 叉分蓼) |
Isoquercetin | 10 |
| Polygonum multiflorum (He Shou Wu, 何首乌) |
Chrysophanol | 15 |
| Physcion | 15 | |
| 2-Methoxystypandrone | 15 | |
| (E)-2, 3, 5, 4′-tetrahydroxystilbene-2-O-(4" -O-a-d-glucopyranosyl)-β-d-glucopyranoside | 31 | |
| (E)-2, 3, 5, 4′-tetrahydroxystilbene-2-O-(6" -O-β-d-glucopyranosyl)-β-d-glucopyranoside | 31 | |
| (E)-2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-d-glucopyranosyl-4′-O- a-d-glucopyranoside | 31 | |
| (E)-2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-d-glucopyranosyl-5-O- a-d-glucopyranoside | 31 | |
| (E)-2, 3, 5, 4′-tetrahydroxystilbene-2-O-(2" -O-β-d-fructofuranosyl)-β-d-glucopyranoside | 31 | |
| Polygonum glabrum. (Guang Liao, 光蓼) |
(2)-2-methoxy-2-butenolide-3-cinnamate | 40 |
| Polygonum hydropiper (Shui Liao, 水蓼) |
Tadeonal | 44 |
| Isotadeonal | 44 | |
| Polygonum hyrcanicum (Hua Nan Liao, 华南蓼) |
Quercetin 3-O-α-l-(3" , 5" -diacetyl arabinofuranoside) | 13 |
| Quercetin 3-O-α-l-(3" -acetyl-arabinofuranoside) | 13 | |
| Quercetin 3-O-α-l-arabinofuranoside | 13 | |
| Myricetin 3-O-α-l-(3" , 5" -diacetyl-arabinofuranoside) | 13 | |
| Myricetin 3-O-α-l-arabinofuranoside | 13 | |
| Polygonum jucundum (Yu Yue Liao, 愉悦蓼) |
3, 5, 7-three hydroxy chromone | 4 |
| Apigenin | 4 | |
| Polygonum lapathifolium (Suan MoYe Liao, 酸模叶蓼) |
Lapathosides A-D | 33 |
| Vanicoside B | 34 | |
| Hydropiperoside | 34 | |
| Polygonum orientale (Hong Liao, 红蓼) |
8-O-β-d-glucopyranosyl-3β, 7β-dihydroxy-lup-20(29)-en-28-oate | 27 |
| Polygonum oriental (Hong Cao, 荭草) |
N-cis-feruloyltyramine | 36 |
| N-trans-feruloyltyramine | 36 | |
| Paprazine | 36 | |
| Polygonum persicaria (Chun Liao, 春蓼) |
5-Hydroxy-7-methoxyflavanone | 7 |
| 6′-Hydroxy-2′, 4′-dimethoxychalcone | 7 | |
| 2′, 6′-Dihydroxy-4′, 5′-dimethoxychalcone | 7 | |
| 2-Carboxy-3, 5-methoxy-E-stilbene | 37 | |
| Polygonum paleaceum (Cao Xue Jie, 草血竭) |
Epicatechin | 9 |
| Procyanidin B-C | 9 | |
| Neopaleaceolactoside | 39 | |
| Polygonum perfoliatum L. (Gang Ban Gui, 杠板归) |
6-Acety1-3, 6-diferuloylsucrose | 19 |
| 2, 4, 6-Triacetyl-3-6-diferuloylsucrose | 19 | |
| 1, 2, 4, 6-Tetraacetyl-3, 6-diferuloylsucrose | 19 | |
| 1, 2, 6-Triacetyl-3, 6-diferuloylsucrose | 19 | |
| 2, 6-Diacetyl-3, 6-diferuloylsucrose | 19 | |
| 8-Oxo-pinoresinol | 20 | |
| 13-Hydroxy helianthemum 8-(17), 14-diene-19-aldehyde | 22 | |
| (24S)-24-Ethylcholesta-3β, 5α, 6α-triol | 23 | |
| Cucurbitacin Ⅱa | 23 | |
| Cucurbitacin U | 23 | |
| Asteryunnanoside F | 23 | |
| Saikosaponin M | 23 | |
| Guanyeliaoine Ⅰ | 45 | |
| Guanyeliaoine Ⅱ | 45 | |
| Polygonum pubescens Blume. (Fu Mao Liao, 伏毛蓼) |
Vanicoside A′ (121) | 46 |
| Hydropiperoside-B (122) | 46 | |
| Hydropiperoside A (123) | 46 | |
| Polygonum runcinatum (Yu Ye Liao, 羽叶蓼) |
3, 3, 4′-Trimethylellagic acid | 41 |
| 3, 3-Di-O-methyl ellagic acid | 41 | |
| 3, 3, 4-Tri-O-methylellagic acid -4′-d-glucopyranoside | 41 | |
| Runcinatside | 38 | |
| 3, 3′-Dimethylellagic acid | 38 | |
| 3, 3′, 4′-Trimethylellagic acid | 38 | |
| 3, 3′-Dimethylellagic acid-4′-O-β-d-glucoside | 38 | |
| 3-Methylellagic acid-4′-O-a-l-rhamno-pyranoside | 38 | |
| Polygonum sieboldii (Jian Ye Liao, 箭叶蓼) |
Tricin | 5 |
| 7, 4-Dimethoxy kaempferol | 5 | |
| Polygonum viscosum (Xiang Liao, 香蓼) |
Iscozulenic acid methylester | 26 |
| Viscoazucinic acid | 26 | |
| Polygosumic acid | 26 | |
| Viscosumic acid | 28 | |
| Polygonum viviparum L. (Zhu Ya Liao, 珠芽蓼) |
Viviparum A | 14 |
| Viviparum B | 14 | |
| Rumex japonicus (Yang Ti, 羊蹄) |
Rhein | 13 |
| Emodin-6-methyl-ether | 13 |