| Citation: | WEI Ke, ZHOU Jia-Hao, CHEN Yong-Chao, TIAN Jia-Ming, SUN Meng-Jin, DAI Xin-Wen, LI Bai-Hui, LI Ling, ZHOU Fang-Liang, NING Yi, HU Jue, XIANG Qin, ZHANG Bo, LU Fang-Guo. Effects of Pachyman in Combination with Vinorelbine and Cisplatin on Tumor Growth and the Expression of EGFR and K-ras in Mice with Lung Cancer[J]. Digital Chinese Medicine, 2018, 1(4): 310-315. |
Lung cancer is the most common cancer in China. Statistical data indicate that the 5-year prevalence of lung cancer from 2006~2011 in China was 130.2 (1/100000). Lung cancer ranks second among malignant tumors in men and fourth among malignant tumors in women [1]. The most common platinum-based chemotherapeutic regimen for lung cancer in China includes administration of cisplatin in combination with vinorelbine, gemcitabine and paclitaxel [2]. Administration of vinorelbine in combination with cisplatin has good clinical efficacy, but this regimen is associated with some adverse effects [3, 4]. Pachyman is the active ingredient in Wolfiporia extensa (Fu Ling, 茯苓) and has antitumor, anti-inflammatory, immunoregulatory and antiviral effects [5]. Many types of polysaccharides from traditional Chinese medicines are used to prevent the development of tumors and increase the susceptibility of tumors to chemotherapy and radiotherapy. Therefore, these agents are often used with chemotherapy and radiotherapy to increase efficacy and decrease toxicity [6-9]. A previous study showed that pachyman is effective in the treatment of tumor due to its low toxic and side effects and high safety. The anti-tumor mechanism of Pachyman is mainly through the regulation of body immunity [6]. To date, however, few studies have investigated the effects of pachyman on the expression levels of tumor-associated genes. Therefore, we constructed a mouse model of lung cancer and investigated the effects of pachyman monotherapy or pachyman in combination with vinorelbine and cisplatin on the expression of epidermal growth factor receptor (EGFR) and K-ras in tumor tissues.
Twenty four 6~8 week old BALB/c SPF mice weighing (20 ± 2) g were purchased from Hunan SJA Laboratory Animal Co. Ltd. (Changsha, China) with equal numbers of male and female mice. The human lung adenocarcinoma cell line A549 were purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences.
Pachyman was purchased from Busky Pharmaceutical Co., Ltd. (Batch number: 20131101). Vinorelbine (NVB) was purchased from Energy Chemical Co. Ltd. (Shanghai; No. E100527; Batch number: GA060070). Cisplatin (DDP) was purchased from Sigma Aldrich Co. Ltd. (Shanghai; Batch number: MKCD4531). Dulbecco's modified Eagle's medium (DMEM) was purchased from Gibco Inc. (USA) and 10% fetal bovine serum (FBS) was purchased from Hyclone Inc. (USA).
A549 cells were cultured in DMEM containing 10% FBS in an incubator at 37℃ and 5% CO2. A549 cells in the logarithmic growth phase were collected and resuspended in serum-free DMEM at a concentration of 1 × 107 cells/mL to create a cell suspension.
The 24 BALB/c mice were allowed to acclimatize for one week. The A549 cell suspension was diluted with DMEM to a concentration of 5 × 106/mL. We subcutaneously injected 0.2 mL A549 cell suspension 0.3 ~ 0.5 cm at the back of the right forelimb axilla.
Stable xenografts were formed on day 15 after inoculated with A549 cells in mice. The model mice were randomly divided into 4 groups as follows: physiological saline group (control group): animals in this group received an intraperitoneal injection of an equal amount of 0.9% physiological saline and simultaneous gavage with 2 mL of sterile distilled water; pachyman group: animals in this group received gavage with 2 mL of 40 mg/mL pachyman and simultaneous intraperitoneal injection of an equal volume of 0.9% physiological saline; vinorelbine + cisplatin group: animals in this group received an intraperitoneal injection of 2.1 mg/kg of vinorelbine and 5 mg/kg of cisplatin and simultaneous gavage of 2 mL sterile distilled water per mouse; pachyman in combination with vinorelbine + cisplatin group: animals in this group received gavage with 2 mL of 40 mg/mL pachyman per mouse and an intraperitoneal injection of 2.1 mg/kg of vinorelbine and 5 mg/kg of cisplatin.
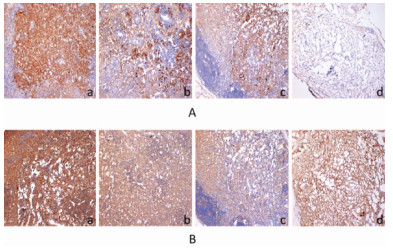
On day 21 after the drugs were administered to the mice, the tumor tissues from the mice were fixed in 4% paraformaldehyde, then, paraffin-embedded sections were obtained according to the standard procedure. Dewaxing, hydration, endogenous catalase removal and antigen retrieval using the standard procedure, and subsequently, the tissue biopsies were incubated with normal goat serum at room temperature for 1 h. The tissue biopsies were incubated at 4 ℃ overnight with EGFR and K-ras primary antibodies. The tissue biopsies were stained, counterstained, dehydrated and dehydrated before all counting. The expression levels of EGFR and K-ras in the tumor tissues from various groups were observed under the microscope using 10 × and 40 × magnification.
TRIzol was used to extract total RNA from tumor tissues and RT-PCR was used to detect the mRNA expression levels of EGFRand K-ras in tumor tissues.
Software Prime 3.0 was used to design primer sequences, which were examined using the NCBI BLAST database. GAPDH, upstream: 5' AGGTCGGTGTGAACGGATTTG 3'; downstream: 5' TGTAGACCATGTAGTTGAGGTCA 3'. EGFR, upstream: 5' GCCATCTGGGCCAAAGATACC 3'; downstream: 5' GTCTTCGCATGAATA GGCCAAT 3'. K-ras, upstream: 5' CAAGAGCGCCTTGACGATACA 3'; downstream: 5' CCAAGAGACAGGTTTCTCCATC 3'. The reaction system was as follows: Taq DNA polymerase activation at 95℃ for 10 min followed by 40 cycles at 95℃ for 15 s and at 60℃ for 1 min. The ABI 7900HT fluorescence quantitative PCR system was used to measure the Ct values of various samples. The mRNA expression levels were calculated using the 2-△△Ct formula.
Statistical software SPSS 22.0 was used for data analysis. P < 0.05 was considered to be significant difference. The data from various groups were expressed as x±s. We performed normality and variance homogeneity tests. One-way analysis of variance (ANOVA) was used for data that showed normal distribution and homogenous variance. The Kruskal–Wallis test was used for data that did not show normal distribution or showed non-homogenous variance.
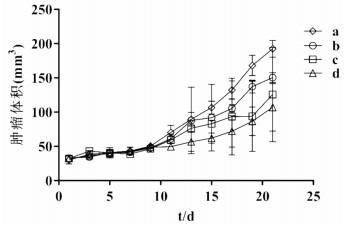
On day 3 after inoculation of A549 cells into the mice, small nodules (measuring approximately 3 × 3mm) could be palpated at the inoculation site. Subsequently, the nodules shrunk in size and then showed a gradual and stable growth from day 9 onwards. On day 15 after inoculation, tumors measuring approximately 4 × 4 mm were formed in all 24 mice. Drugs were administered on day 15 after inoculation. Vernier caliper was used to measure the longest diameter (a), shortest diameter (b), and tumor volume V=ab2/2 every 2 days after drug administration, and we plotted a curve depicting the 21-day variation in tumor volume since drug administration. The results showed that the growth rate of the tumors of mice in the physiological saline control group was significantly higher than that of the mice in other groups (Fig. 1). Pachyman combined with vinorelbine and cisplatin had stronger tumor inhibition than other groups.
Results of immunohistochemical staining showed that EGFR and K-ras were significantly expressed in all 4 groups and were widely distributed in the cytoplasm and cell membrane of tumor cells (Fig. 2). The software IPP 6.0 was used to scan and analyze the photos of each group (Fig. 2). Compared to the control group, the vinorelbine + cisplatin group showed a decrease in the tissue expression levels of EGFR and K-ras. In addition, pachyman showed an enhanced effect in decreasing the expression and distribution of EGFR and K-ras in lung cancer tissues in model mice (Fig. 3).
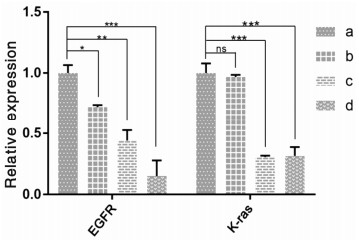
RT-PCR was used to determine the relative mRNA expression of EGFR and K-ras in the tumor tissues from various group mice. Vinorelbine and cisplatin significantly decreased the mRNA expression levels of EGFR and K-ras (P < 0.001) (Fig. 4). Pachyman monotherapy significantly decreased the expression of EGFR in lung cancer tissues (P < 0.01). In addition, compared to administration of vinorelbine + cisplatin alone, administration of pachymanin combination with vinorelbine + cisplatin showed a significant decrease in the expression of EGFR (P < 0.0001). However, pachyman monotherapy did not show a significant decrease in the mRNA expression levels of K-ras (P > 0.1). Compared to vinorelbine + cisplatin, pachymanin combination with vinorelbine + cisplatin did not induce a significant decrease in the mRNA expression levels of K-ras.
Lung cancer is a malignant tumor with high mortality rates worldwide and is mainly classified as non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). More than 80% of lung cancers are NSCLCs [10]. Dual drug regimens combining a platinum-based drug and a third-generation drug are standard regimens for the treatment of NSCLC. Statistical data show that the efficacy and safety of first-line regimen with vinorelbine and cisplatin needs to be improved [11-13]. Pachyman is derived from the W. extensa and has immunity boosting and antitumor effects [14-16]. The current study showed that pachyman could increase the chemotherapeutic effects of vinorelbine and cisplatin, and decreased the formation of NSCLC tumors that in model mice of NSCLC constructed using A549 cells.
EGFR is a common tyrosine kinase receptor that belongs to the ErbB family. EGFR is highly expressed in lung cancer cells, and the expression level of this gene is intimately associated with proliferation, invasion and metastasis of lung cancer cells. EGFR has become one of the main targets of research for developing drugs against lung cancer. The K-ras gene is an important signaling molecule in downstream of the EGFR pathway, and the changes in the K-ras expression levels are positively correlated with sensitivity EGFR agonists [17-18]. Cisplatin-based dual chemotherapy regimens decrease the expression of EGFR and increase cytotoxicity towards A549 cells [19, 20]. In addition, EGFR expression levels are positively correlated with the sensitivity of tumor cells to vinorelbine [21]. Our results showed that both EGFR and K-ras were highly expressed in NSCLC tumor tissues, and vinorelbine and cisplatin significantly decreased the mRNA expression levels of EGFR and K-ras in NSCLC tumor tissues. Pachyman monotherapy or combination therapy with vinorelbine + cisplatin significantly reduced the mRNA expression levels of EGFR but did not significantly decrease the mRNA expression levels of K-Ras. Thus, our results showed that the mechanism of the effect of pachyman in increasing the efficacy of vinorelbine + cisplatin in the treatment of NSCLC is associated with the EGFR but not with the EGFR—K-ras signaling pathway and dephosphorylation may decrease the stability of the K-ras protein. Further detailed studies are required to elucidate the molecular mechanisms responsible for the effects of pachyman in lung cancer.
We thank for the funding support from the National Natural Science Foundation of China (No. 81774126, No. 81503445 and No. 81703919), China Postdoctoral Science Foundation (No. 2017M622587), Hunan Provincial Natural Science Foundation (No. 2016JJ2095 and No. 2017JJ3232), Hunan Provincial Traditional Chinese Medicine Key Research Project (No.201701), Hunan Education Department Scientific Research Project (16C1203 and 16C1216), Hunan Health Department Scientific Research Project (C2015-16, C2016049 and B2016093), Busky Pharmaceutical Collaborative Research Fund, Hunan Provincial Higher Educational Institutions Research Team "Traditional Chinese Medicine prevention and treatment research on infectious diseases" Funding program (No. 15), and Hunan Province Teaching and Science "Thirteenth Five-Year Plan" Project (No. XJK17BGD057).
The authors declare no conflict of interest.
| [1] |
SHI Y K, SUN Y, YU J M, et al. China experts consensus on the diagnosis and treatment of advanced stage primary lung cancer (2016 version). China J Lung Cancer, 2016, 19(1):1-15. http://www.ir.xjtu.edu.cn/jspui/item/ir/400165
|
| [2] |
XUE C, HU Z, JIANG W, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer, 2012, 77(2): 371-375. doi: 10.1016/j.lungcan.2012.04.014
|
| [3] |
FANG A P, WANG J P, HE Y F, et al. The efficacy and toxicity of vinorelbine combined with cisplatin in the treatment of NSCLC. Contemporary Medicine, 2016, 22(14):146-147. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zhzl200904015
|
| [4] |
WOOD D E, KAZEROONI E A, BAUM S L, et al. Lung cancer screening, version 3. 2018, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network: JNCCN, 2018, 16(4):412-441. doi: 10.6004/jnccn.2018.0020
|
| [5] |
ZHU G X, LI Y Q. The research progress about the regulation of pachyman in immunoregulation. Nutrition and Feed, 2018, 35(6):80-81. http://cn.bing.com/academic/profile?id=32d5a38b35c8034dbc7d12b66823e34c&encoded=0&v=paper_preview&mkt=zh-cn
|
| [6] |
YANG H J, TIAN D, LIU Z X, et al. Effect study of poria polysaccharide oral solution with oxaliplatin + capecitabine on quality of life and immune function in the treatment of patients with advanced gastric cancer. Medical Innovation of China, 2017, 14(17): 23-26. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgyxcx201717006
|
| [7] |
LIU S X, CHEN M C, JIANG B H, et al. Efficacy analysis of Astragalus polysaccharide injection combined with EOF regimen in the treatment of advanced gastric cancer. Chin J Integr Trad West Med Dig, 2014, 22(11): 684-686. http://www.ncbi.nlm.nih.gov/pubmed/24998543
|
| [8] |
YIN H, LIN Y G. Efficacy of synergistic combination of lentinan and GP regimen in stage Ⅳ lung squamous cell carcinoma. Oncology Progress, 2016, 14(7): 687-689. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=azjz201607024
|
| [9] |
GE M J, YU M, CAO X Y. Study on the effectiveness and safety of ginseng polysaccharide adjuvant GP chemotherapy in the treatment of nonsmall cell lung cancer. Chinese Journal of Biochemical Pharmaceutics, 2015, 35(4): 132-134. doi: 10.1002/1097-0142(19950901)76:5<779::AID-CNCR2820760511>3.0.CO;2-O/pdf
|
| [10] |
SIEREL R L, MILLER K D, JEMAL A. Cancer statistics, 2018. CA Cancer J Clin, 2018, 68(1):7-30. doi: 10.3322/caac.v68.1
|
| [11] |
LIU T S, WU H, ZHUANG X D, et al. A Meta-analysis of platinum plus docetaxel or vinorelbine in the first-line treatment of advanced non-small cell lung cancer. Chin J Lung Cancer, 2014, 17(4):327-335. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_b31120fe26ecaf023be6d8c31819573c
|
| [12] |
LI G, ZHANG S B. Observation of the efficacy of vinorelbine combined with cisplatin in the treatment of advanced non-small cell lung cancer. China Modern Doctor, 2016, 10(30): 90-92. http://en.cnki.com.cn/Article_en/CJFDTotal-ZDYS201630028.htm
|
| [13] |
MORIMOTO Y, TAKEI H, TACHIBANA K, et al. Role of Pharmacists in Completion of Adjuvant Cisplatin-Vinorelbine Chemotherapy in Japanese Patients with Non-small Cell Lung Cancer. Yakugakuzasshi: Journal of the Pharmaceutical Society of Japan, 2018, 138(3): 437-442. doi: 10.1248/yakushi.17-00192
|
| [14] |
HOU W T, LUO J B. The study of the compound poria polysaccharide oral liquid on the antitumor activityand immune regulation function. Pharmacology and Clinics of Chinese Materia Medica, 2017, 33(2):78-81. http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZYYL201702022.htm
|
| [15] |
CHEN L, CUI P W, LU X B, et al. Study on regulation of triterpenoids biosynthesis in Wolfiporia cocos submerged fermentation system by methyl jasmonate. Journal of Hunan University of Chinese Medicine, 2017, 37(6):606-610. http://en.cnki.com.cn/Article_en/CJFDTOTAL-HNZX201706007.htm
|
| [16] |
ZHANG M X, ZHANG D S, ZHUANG P W, et al. Effects of pachymaran on hematogeneous lung metastasis in mice with melanoma B16. Tianjin Journal of Traditional Chinese Medicine, 2014, 31(2): 98-101. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=tjzy201402010
|
| [17] |
ELAMINYY, GOMEZ DR, ANTONOFF MB, et al. Local consolidation therapy (LCT) after first line tyrosine kinase inhibitor (TKI) for patients with EGFR mutant metastatic non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 2018 Sep 24. DOI: https://doi.org/10.1016/j.cllc.2018.09.015.
|
| [18] |
WANG X N, TIAN F. Traditional Chinese medicine combined with EGFR-TKIs in treatment of non-small cell lung cancer: a meta-analysis. Journal of Hunan University of Chinese Medicine, 2018, 38(7):781-786. http://d.old.wanfangdata.com.cn/Periodical/hnzyxyxb201807015
|
| [19] |
XU M, MEI J ZH, LI R J, et al. Killing effect of paclitaxel and cisplatin on EGFR-wild and mutant lung adenocarcinoma cells and related molecular mechanism. Cancer Res Prev Treat, 2016, 43(3):197-200. http://d.old.wanfangdata.com.cn/Periodical/zlfzyj201603006
|
| [20] |
CHEN C, FANG Q, ZHOU Q Y, et al. Effect of S-1+ cisplatin and gemcitabine + cisplatin on malignant indexes of serum and tumor tissue in advanced NSCLC patients with wild-type EGFR. Journal of Hainan Medical University, 2016, 22(15):1710-1713. http://d.wanfangdata.com.cn/Periodical/hnykdxxb-e201615029
|
| [21] |
NING Y L, XIE Y W, CHEN J, et al. Correlation between EGFR Expression on Breast Cancer and NVB Sensitivity. Chinese Journal of Cell Biology, 2014, 36(3):343-348. http://en.cnki.com.cn/Article_en/CJFDTotal-XBZZ201403009.htm
|
| 1. | Liu S., Liu J., Cheng B. et al. Antitumor of Main Active Components of Poria: A Review. Chinese Journal of Experimental Traditional Medical Formulae, 2023, 29: 257-263. DOI:10.13422/j.cnki.syfjx.202202121 |
| 2. | Kaiqin C., Ke W., Chun Y. et al. Immunomodulatory effect of pachymaran on cyclosporine A (CsA)-induced lung injury in mice. Digital Chinese Medicine, 2022, 5: 222-232. DOI:10.1016/j.dcmed.2022.06.011 |
| 3. | Hiengrach P., Visitchanakun P., Finkelman M.A. et al. More Prominent Inflammatory Response to Pachyman Than to Whole‐Glucan Particle and Oat‐β‐Glucans in Dextran Sulfate‐Induced Mucositis Mice and Mouse Injection through Proinflammatory Macrophages. International Journal of Molecular Sciences, 2022, 23 DOI:10.3390/ijms23074026 |